Chemistry - Gas Laws - Unit 9 ✅
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
U9: Ideal Gas Postulates
1) the volume of gas particles is so small compared to the distance between them that the volume is assumed to be negligible
2) gas particles exhibit NO INTERMOLECULAR INTERACTION with their surrouding or other gas molecules/particles
3) gas particles are in constant, random, rapid straight lines of motion, colliding with eachother and the sides of the container
4) gas particles have elastic collisions with eachother and the walls of the container (conservation of energy)
U9: What does each ideal gas postulate contradict
Negligible volume = matter has mass, its small but there
No IMFS = All molecules are some type of weak London dispersion
Constant, random, straight line movement = gravity and magnetism can cause predicted and bent movement
Total elastic collisions = a small amount of energy is lost with collision
U9: Ideal Gases
Real gases behave most like ideal gases under high temperatures and low pressure
U9: Pressure of gas
Measuring the collisions of gas particles within a container
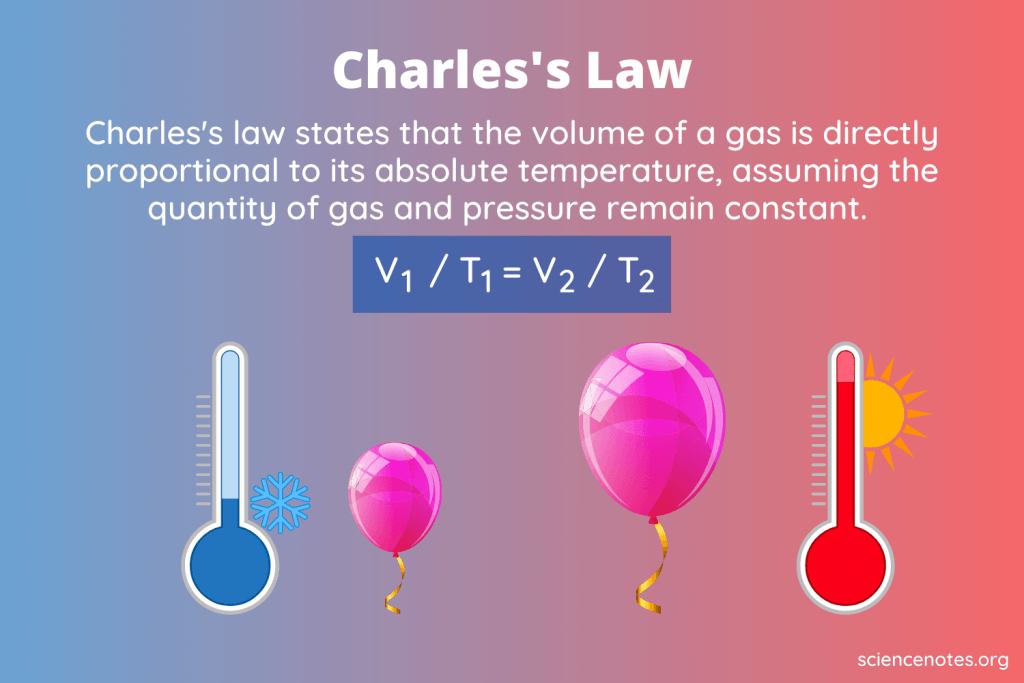
U9: What is the relationship between temperature and volume
Linear
As temperature decreases, volume decreases
As volume increases, temperature increases
V1/T1=V2/T2 (both go up or both go down)
Always use KELVIN!
U9: What is the relationship between temperature and pressure?
Gay-Lussacs Law (constant volume)
Direct, linear relationship
Temp increase, pressure increase and temp decrease, pressure decrease
Why? Temp increase = more kinetic energy = more collisions = higher pressure
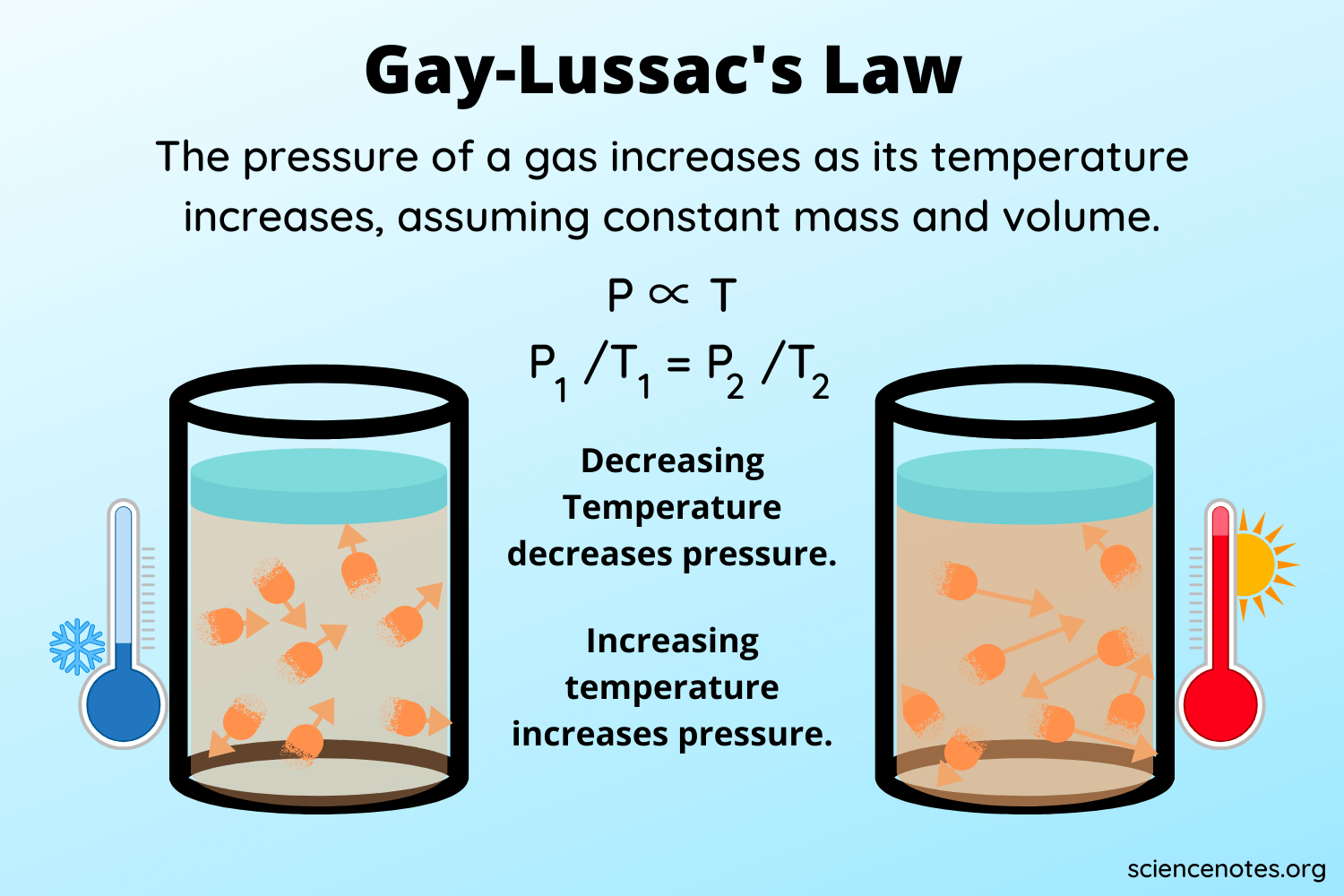
U9: Before doing any equations you must..
Convert to kelvin and make sure the units are the same (both atp or both kPa)
U9: What gases are most likely to act like ideal gases?
Hydrogen and Helium
U9: Can volume be negative (absolute zero)
NO NEVER! This is because we never know the particles exact location and/or movement
Thus, we MUST use kelvin because Celsius will give us 0
U9: Pressure
Force applied to an object by another object
Pressure = force/area
Units= atmospheres (kPa) or kilopascals (kPa)
1 atm=101.3kPa
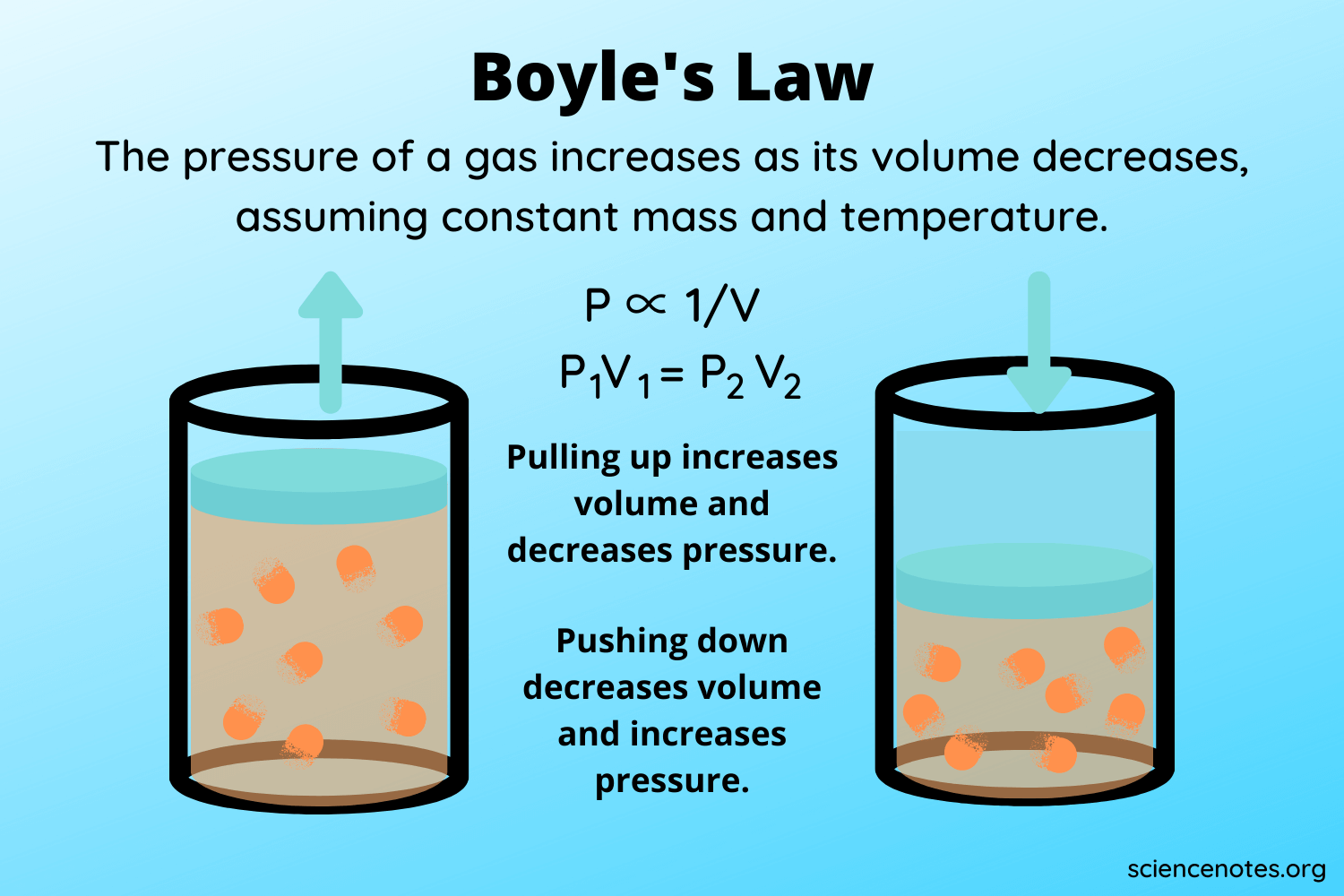
What's the relationship between pressure and volume?
BOYLES LAW
—> INVERSE
As pressure decreases, volume increases. Constant temp.
This is because: air inside can expand with less pressure and take up more volume ex: balloon
Note: THIS IS AN EXPONENTIAL RELATIONSHIP meaning it's a more sloped relationship if graphed and not a straight line
VOLUME AND PRESSURE NEVER REACH ZERO
P1xV1 = P2xV2
Avogadros Law ⭐️
At constant temperature
V1/n1 = V2/n2
At standard STP (temp and pressure) the volume of a gas is directly related to the number of particles in the container
The volume the moles occupies does not differ between substances.
1mole = 22.4L (IDEAL gas)
How much volume does one mole take up ⭐️
22.4L
When does the number of gas molecules/particles change?
ONLY if gas is added or removed
Kinetic Molecular Theory
Constant random motion:
Gas particles are in constant, random motion, moving in straight lines until they collide with other particles or the container walls.
Negligible particle volume:
The combined volume of the gas particles is negligible compared to the volume of the container they occupy.
No intermolecular forces:
Gas particles exert no attractive or repulsive forces on one another except during collisions.
Elastic collisions:
Collisions between gas particles and the container walls are perfectly elastic, meaning no kinetic energy is lost during collisions.
Average kinetic energy proportional to temperature:
The average kinetic energy of the gas particles is directly proportional to the absolute temperature of the gas.
What conditions are needed for the same number of particles?
Same volume, pressure, temp (Avogadro)
Under which conditions of pressure and temp is a real gas like an ideal gas ⭐️
Low pressure and high temp
Or small particles/radius