IGCSE Chemistry Ion and Gas Tests
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Potassium (K+) ions
Lilac Flame

Lithium (Li+) ions
Red flame

Sodium (Na+) ions
Yellow flame

Calcium (Ca2+) ions
Red-Orange flame

Copper (Cu2+) ions
Green flame

Iron (Fe2+) ions
Green PPT (with NaOH)

Iron (Fe3+) ions
Brown PPT (with NaOH)

Copper (Cu2+) ions
Blue PPT (with NaOH)

Sulfate (SO4 2-) ions
White PPT (with acidified Barium Chloride)

Carbonate (CO3 2-) ions
Gas produced (when reacted with acid) turns limewater cloudy
Chloride (Cl-) ions
White PPT (with acidified Silver Nitrate)

Bromide (Br-) ions
Cream PPT (with acidified Silver Nitrate)

Iodide (I-) ions
Yellow PPT (with acidified Silver Nitrate)

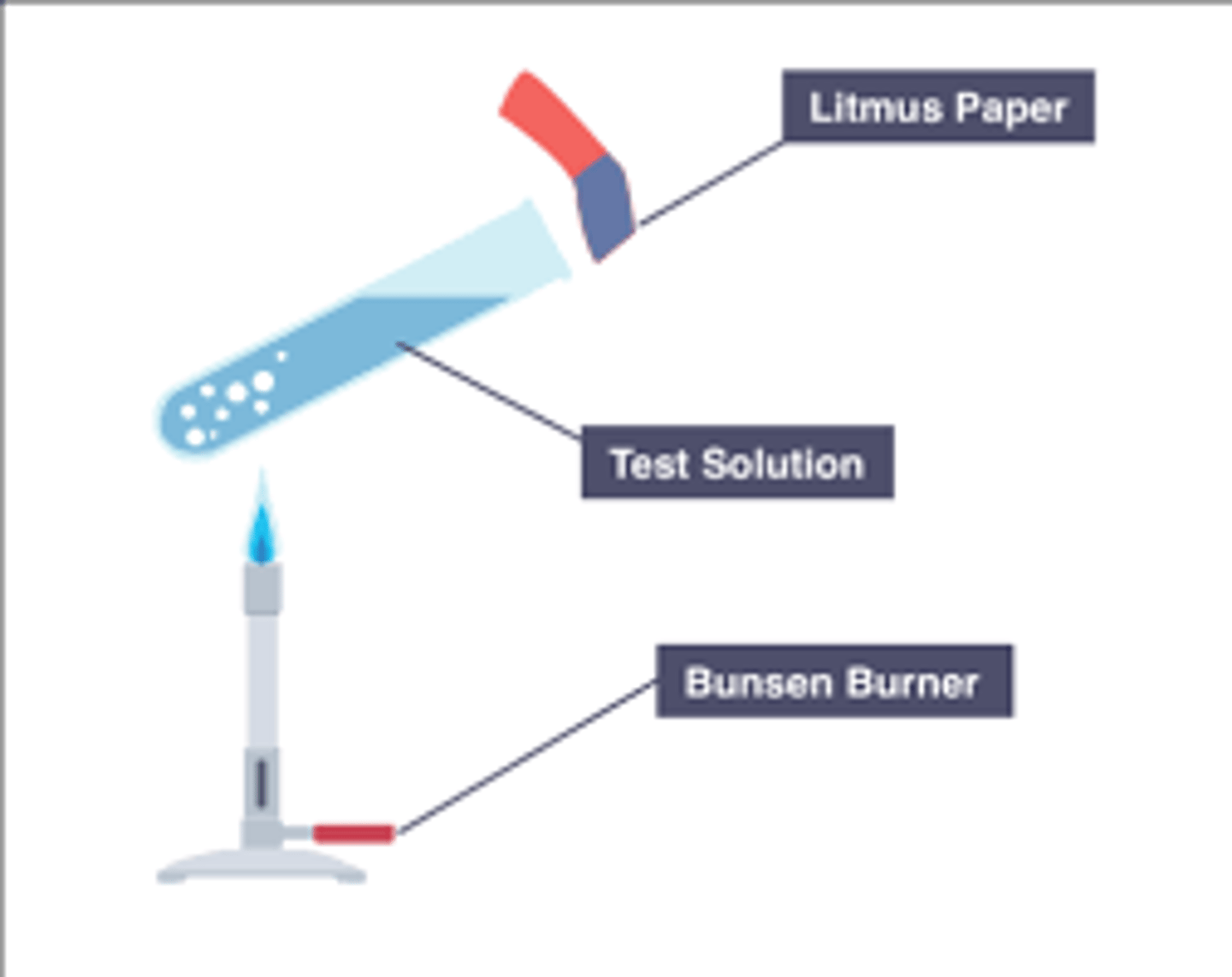
Ammonium (NH4+) ions
Gas produced (when warmed with NaOH) turns red litmus paper blue
Oxygen (O2) gas
Relights a glowing splint

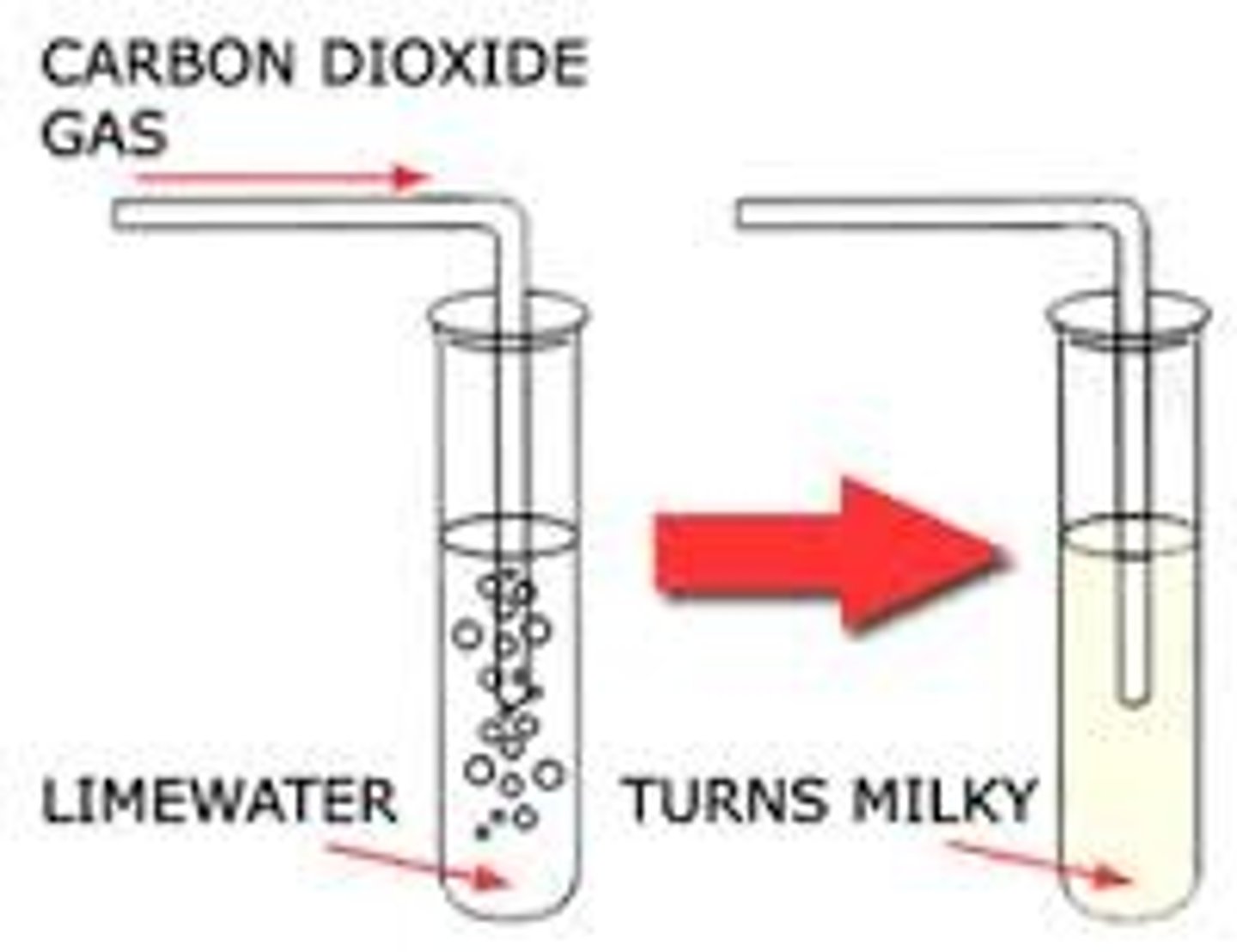
Carbon Dioxide (CO2) gas
Turns limewater milky/cloudy

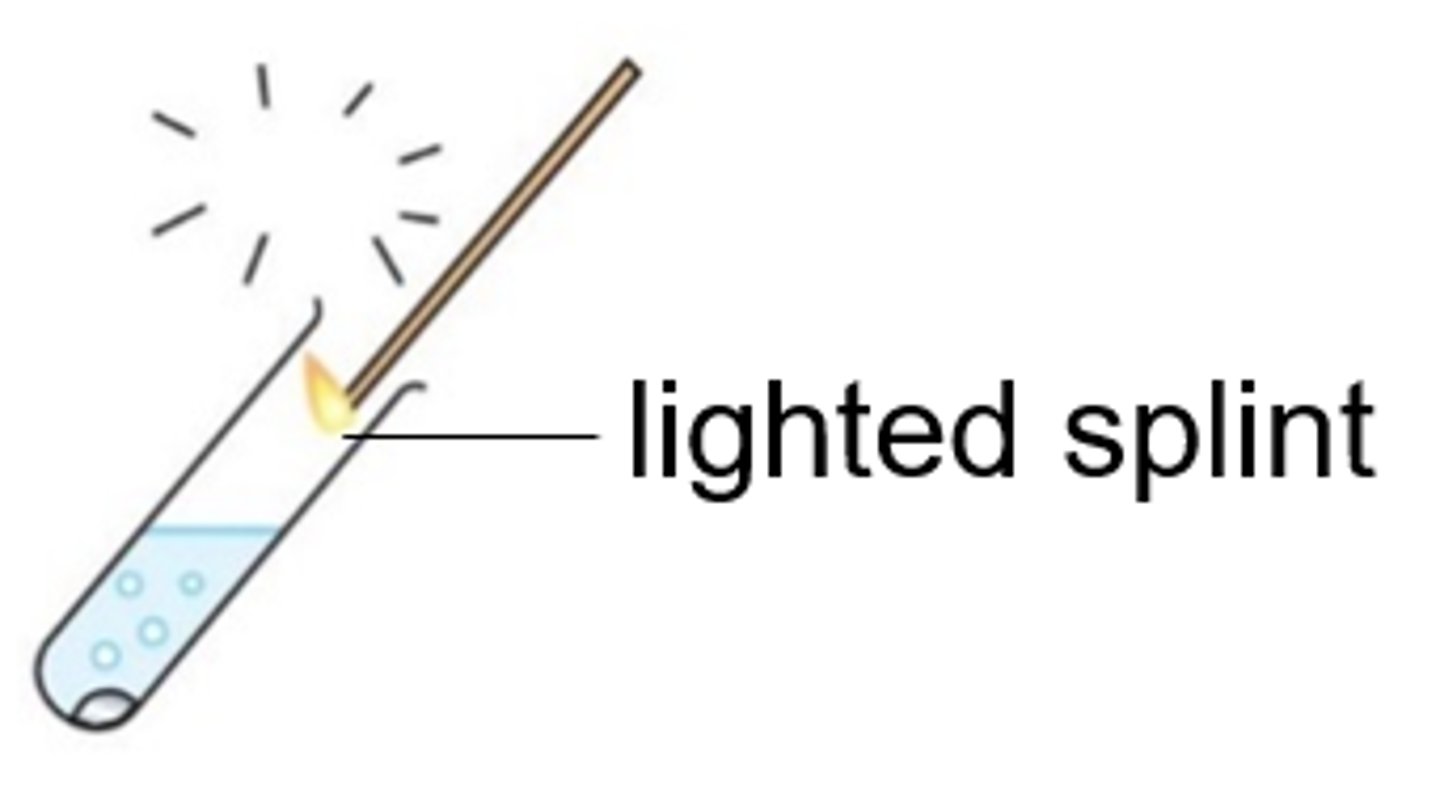
Hydrogen (H2) gas
Lit splint produces a squeaky pop
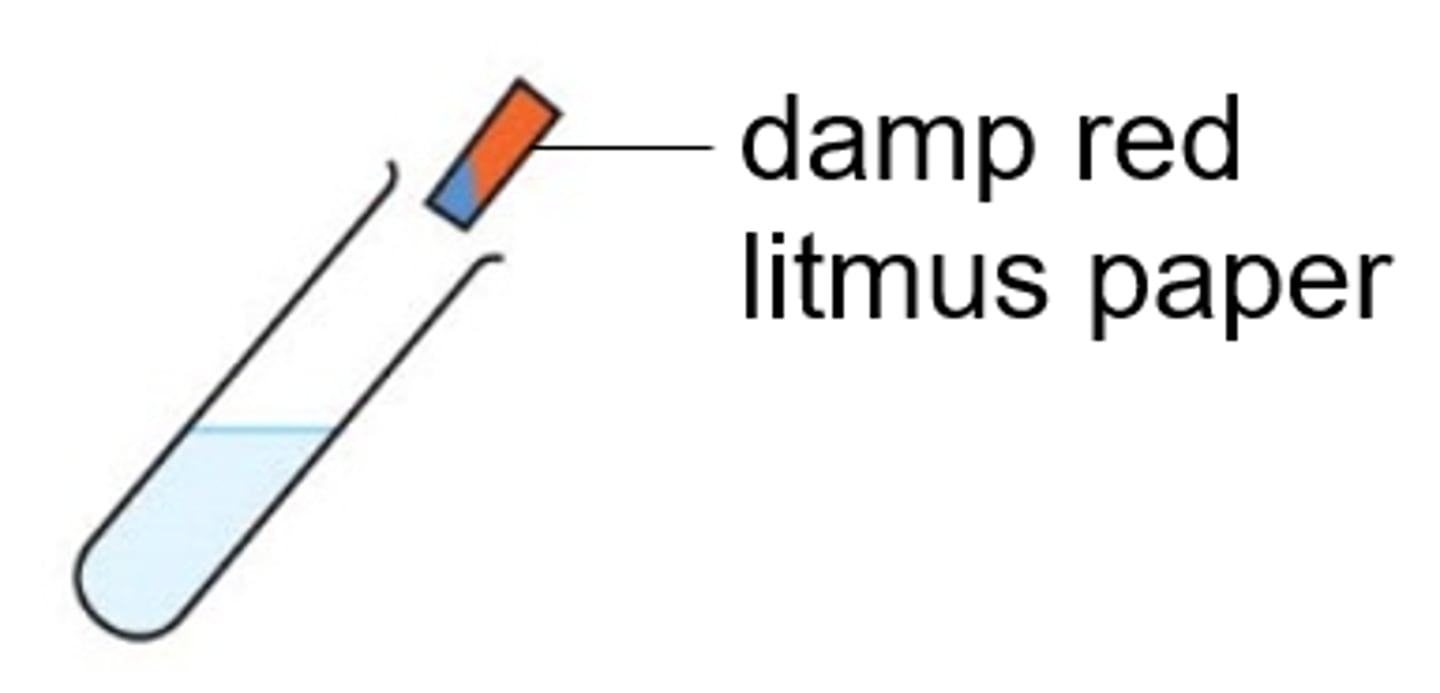
Ammonia (NH3) gas
Moist red litmus paper turns blue
Chlorine (Cl2) gas
Moist blue litmus paper turns red then white (bleaches)

Chemical Test for Water
Anhydrous Copper Sulfate turns from White to Blue

Physical Test for pure water
boils at 100 degrees, freezes at 0 degrees
Limewater is a solution of ...
Calcium Hydroxide
The reason limewater turns cloudy with Carbon Dioxide is because _____________ is formed?
Calcium Carbonate