4.3 Alcohols and Phenols
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Nucleophiles
ions or compounds possessing a lone pair of electrons that can seek out a relatively positives site
donates a pair
Eg. -OH, -CN, NH2
Primary, secondary and teritary alcohols
P- Only one alkyl group attached to the carbon hydroxide group
S- 2
T- 3
Electronegativity
Ability of an atom in a chemical bond to polarise electron density towards itself
Difference in boiling points between alcohols and alkanes
set up between
In alcohol there are hydrogen bonds set up between the slightly positive hydrogen and lone pairs on oxygen in other molecules
in alkanes the only intermolecular forces are vander waals dispersion forces hydrogen bonds are much stronger and take more energy to separate alcohol molecules
Forming primary and secondary alcohols from halagenoalkanes
refluxing
Carried out by refluxing together the halogenoalkane and an aqueous solution of an alkali (Na or KOH)
during reflux the liquid is evaporated, cooled and condensed to continue the reaction
separated at the end of the reaction by fractional distillation from diff boiling points
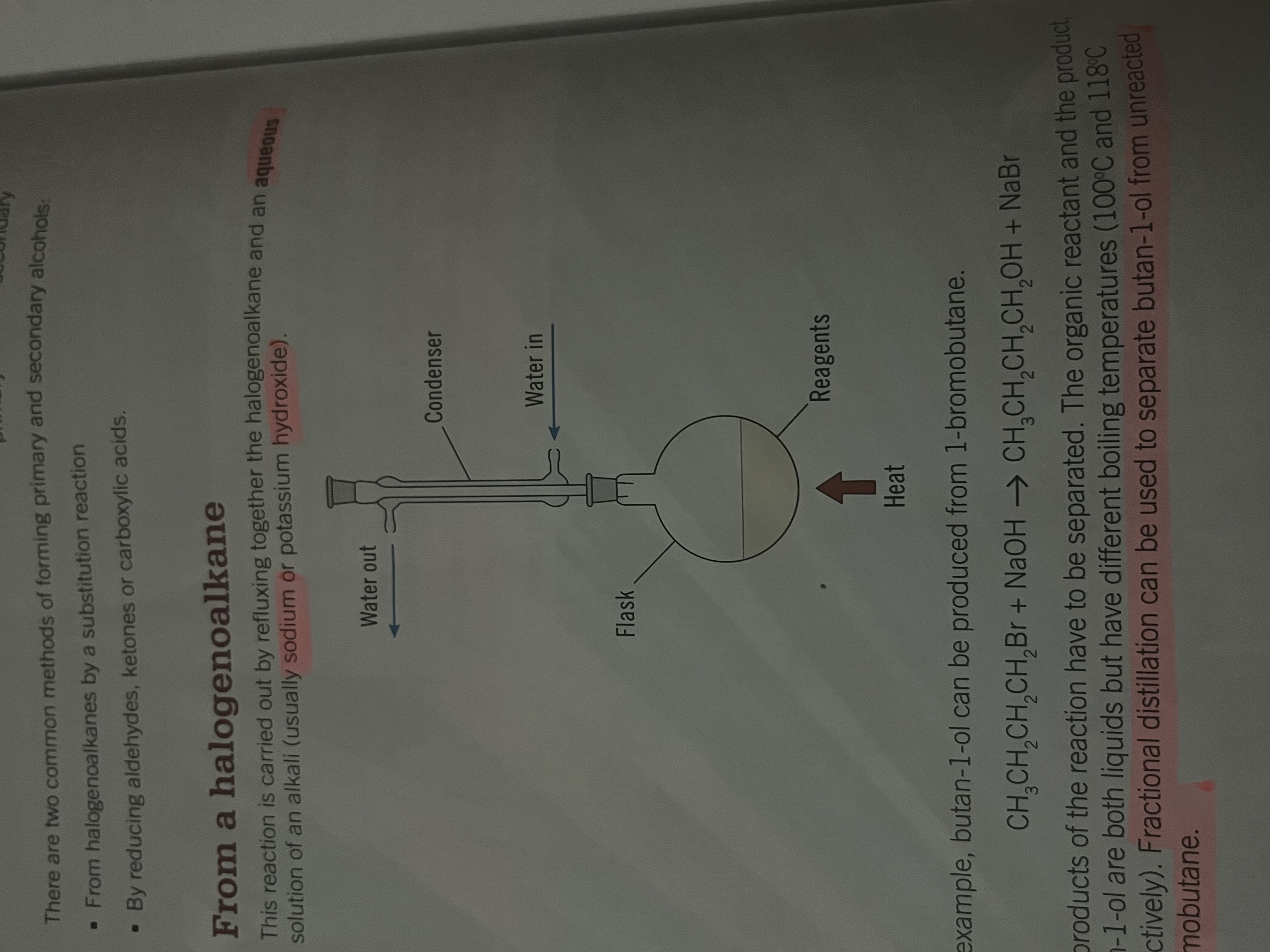
Forming a primary alcohol Eg. Butan-1-ol from 1-bromobutane
substitution
CH3CH2CH2CH2Br + NaOH → CH3CH2CH2CH2OH + NaBr
nucleophilic substitution where the hydroxide ion acts as the nucleophile and attacks the relatively positive carbon atom on the C-Br bond

secondary alcohol formation example
Rate of hydrolysis for alcohols from halogenoalkanes
C—I > C—Br > C—Cl
yield of primary alcohols is usually higher than the yield of secondary alcohols
when producing secondary alcohols a alkene can be formed as a side product
greater yields of alkenes are produced if high concentrations of the alkali are used or if an ethanolic solution of the alkali
Formation of alcohols from the reduction of a carbonyl compound
aldehydes and ketones
Both aldehydes and ketones can be reduced to primary and secondary alcohols by using an aqueous solution of sodium tetrahydridoboarate (NaBH4)
use [H] to represent a reducing agent
![<p>Both aldehydes and ketones can be reduced to primary and secondary alcohols by using an aqueous solution of sodium tetrahydridoboarate (NaBH4)</p><p>use [H] to represent a reducing agent</p>](https://knowt-user-attachments.s3.amazonaws.com/9ed5fb03-1dea-4e74-9885-b7093c9be509.jpg)
Reducing carboxylic acids
dissolved in
Sodium tetrahydridoboarate is not powerful enough to reduce carboxylic acids and the stronger reducing agent lithiumtetrahydridoaluminate (LiAlH4)
Dissolved in ethoxyethane

Reactions with the reducing agents
excess
Sodium tetrahydridor borate is a much safer reaction as an excess of lithium tetrahydridaluminate is difficult to dispose and the solvent ethoxyethane is very flammable
To obtain a good yield of alcohol
the organic solvent
An access of the reducing agent is used
the excess reducing agent can be removed by adding a dilute acid
the organic products needs to be separated from the aqueous mixture
carried out using a separating funnel if the alcohol produced is immisciple with water or separated by adding an organic solvent such as ethoxyethane by the technique of solvent extraction
Reaction of primary and secondary alcohols with hydrogen halides
Halogenoalkanes are produced by reacting the alcohols with hydrogen halides
the reactions are generally slow and reversible often giving poor yields

Chlorination
Passes hydrogen chloride gas into the alcohol in the presence of anhydrous zinc chloride, acting as a catalyst
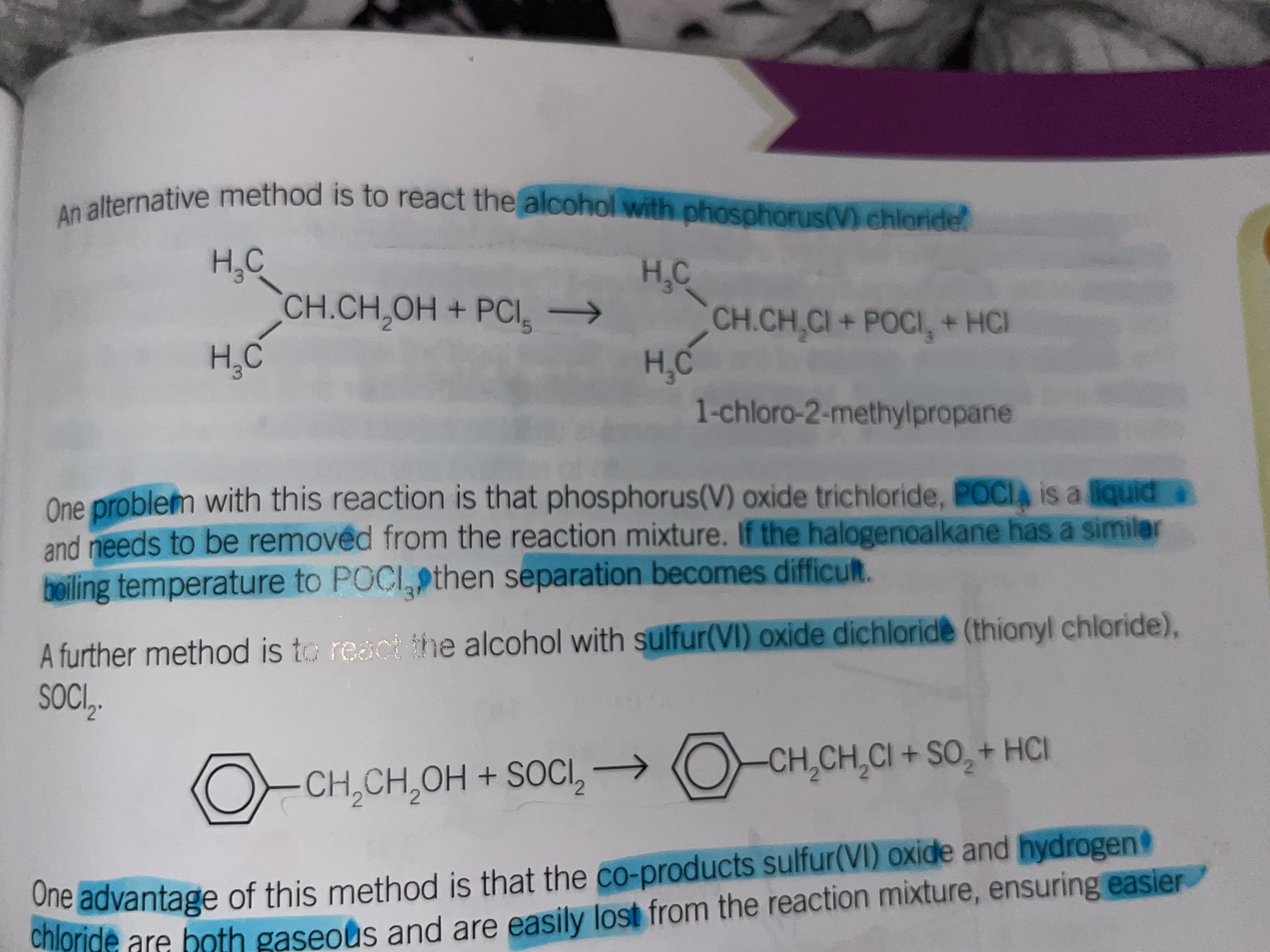
Chlorination using phosphorus chloride (V)
Room temperature
one problem with this reaction is that phosphorus oxide trichloride (POCl3) is a liquid and needs to be removed from the reaction mixture. if the halogenoalkane has a similar boiling temperature to POCl3 then separation becomes difficult

Chlorination using sulphur (VI) oxide dichloride SOCl2
An advantage to this method is that the co products sulphur oxide and hydrogen chloride are both gaseous and are easily lost from the reaction mixture, ensuring easier separation
Bromination
‘in situ’ reaction: potassium bromide and 50% sulfuric (VI) acid is heated
the sulfuric acid protonates the alcohol and this then reacts with the bromide ions from the potassium bromide
CH3CH2CH2CH2OH + KBr + H2SO4 → CH3CH2CH2CH2Br + KHSO4 + H2O
Iodination
warm d
Warm dump red phosphorus and iodine together to form phosphorus iodide (III) PI3
2P + 3I2 → 2Pl3
3CH3CH2CH2OH + PI3→ 3CH3CH2CH2I + H3PO3
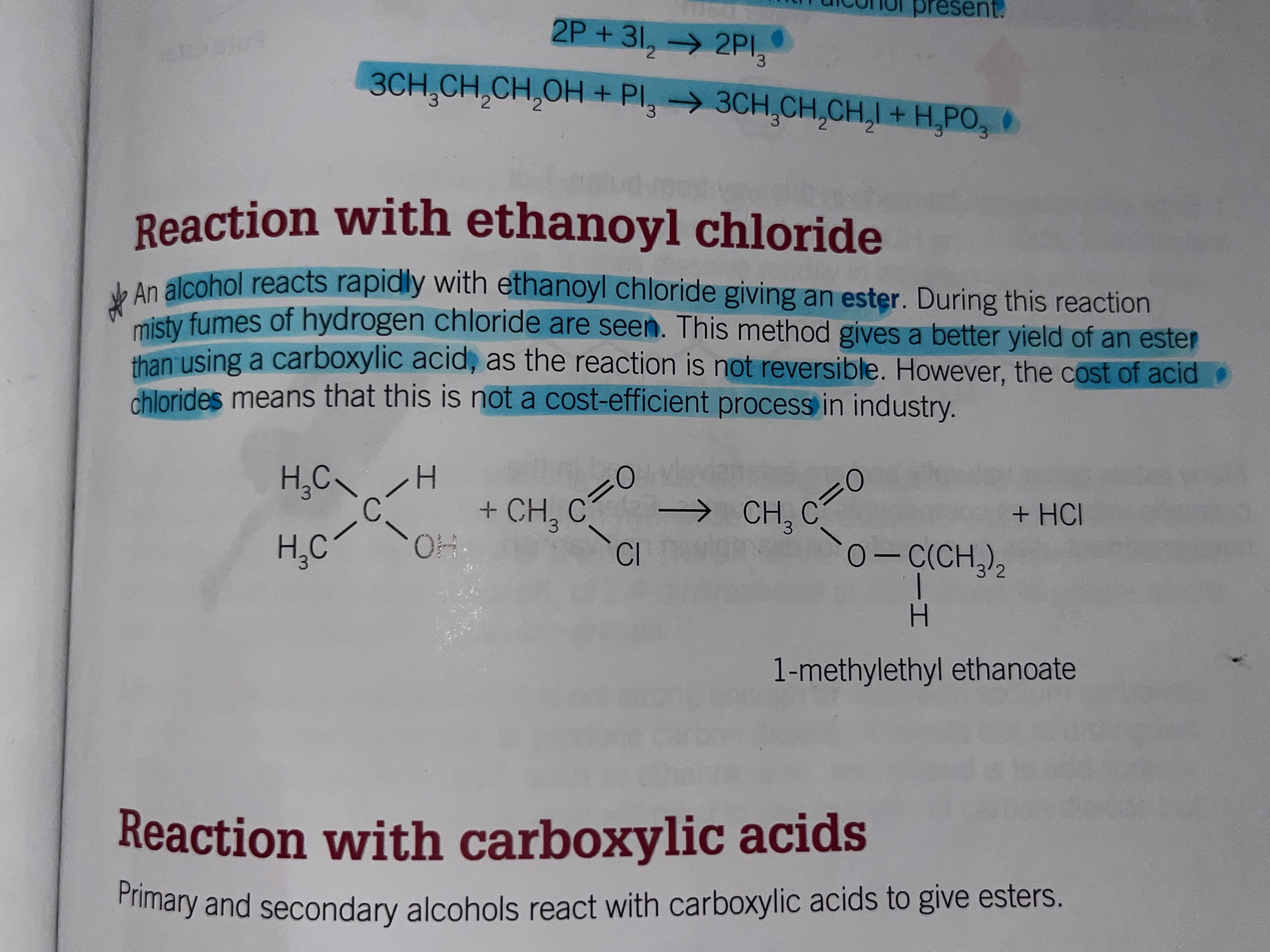
Reaction with carboxylic acids
Primary and secondary alcohols react with carboxylic acids to give esters
Alcohol + COOH → ester + H2O
the reaction is reversible and eventually the mixture will reach a position of equilibrium
To increase the yield of Esters
distilled
Little concentrated sulfuric (VI) acid is added to the mixture of the alcohol and the carboxylic acid, the mixture is heated under reflux
the products can then be distilled and the Ester collected at its boiling temperature
the distillate generally consists of the Ester and water together with a little unreacted alcohol and carboxylic acid. Many esters are immiscible with water and the distillate often consists of two layers
Separating funnel used to extract the Ester
dried
Then shaken with sodium hydrogen carbonate solution to remove any remaining carboxylic acid
Ester is then dried with anhydrous calcium chloride which reacts with any remaining alcohols
it can then be redistilled to give a pure product
Reactions with ethanoyl chloride
Alcohol reacts rapidly with ethanyol chloride giving an ester. During this reaction misty fumes of hydrogen chloride are seen.
Gives a better yield of an Ester than using a carboxylic acid as the reaction is not reversible
however the cost of acid chloride means this is not a cost efficient process in industry
Reactivity of phenols compared to alcohols
lone pair overlap
extended delo
Reactivity is very different
One of the lone pairs of oxygen atoms overlap with the delocalized Pi system to form a more extended delocalized system.
As a result the C—O bonds in phenols is shorter and stronger than in alcohols
making C—O bond fission in phenols harder than co-bond in alcohols
the extended delocalization creates a higher electron density in the Ring and makes the ring structure more susceptible to attack by electrophiles
Acidity of phenol
Phenol is a very weak acid and the position of the equilibrium lies to the left
phenol can lose a hydrogen ion because the phenoxide ion formed is stabilized to some extent. The negative charge on the oxygen atom is delocalized around the ring
The more stable the ion the more likely it is to form
Why is phenol only a weak acid
one of the lone
One of the lone pairs on the oxygen atom overlap with the delocalized electrons on the benzene ring
this overlap leads to a delocalization which extends from then ring out over the oxygen atom resulting in the negative charge no longer entirely localised on the oxygen, but is spread out around the whole ion
spreading the charge around makes the ion more stable
oxygen is the most electronegative element in the ion and the delocalized electron will be drawn towards it. This means that they will be still a lot of charge around the oxygen
which will tend to attract the hydrogen ions back again
Phenol with the sodium hydroxide
gives a
Gives a colourless solution containing sodium phenoxide- showing it must be acidic
hydrogen ion has been removed by the strongly basic hydroxide ion in the solution
this shows phenol reacting as an acid losing a proton to the aqueous hydroxide ion
The presence of substituents on the benzene ring affects the acidity of phenol

phenol tion with sodium carbonate/ sodium hydrogen carbonate
Phenol is not strong enough to react to produce carbon dioxide. No bubbles are produced
shows it's only a weak acid
Phenol with metallic sodium
slow
Slow reaction because phenol is such a weak acid
phenol is warmed in a dry tube until it is molten and a small piece of sodium is added there is some fizzing as hydrogen gases given off
mixture is left in the tube will contain sodium phenoxide
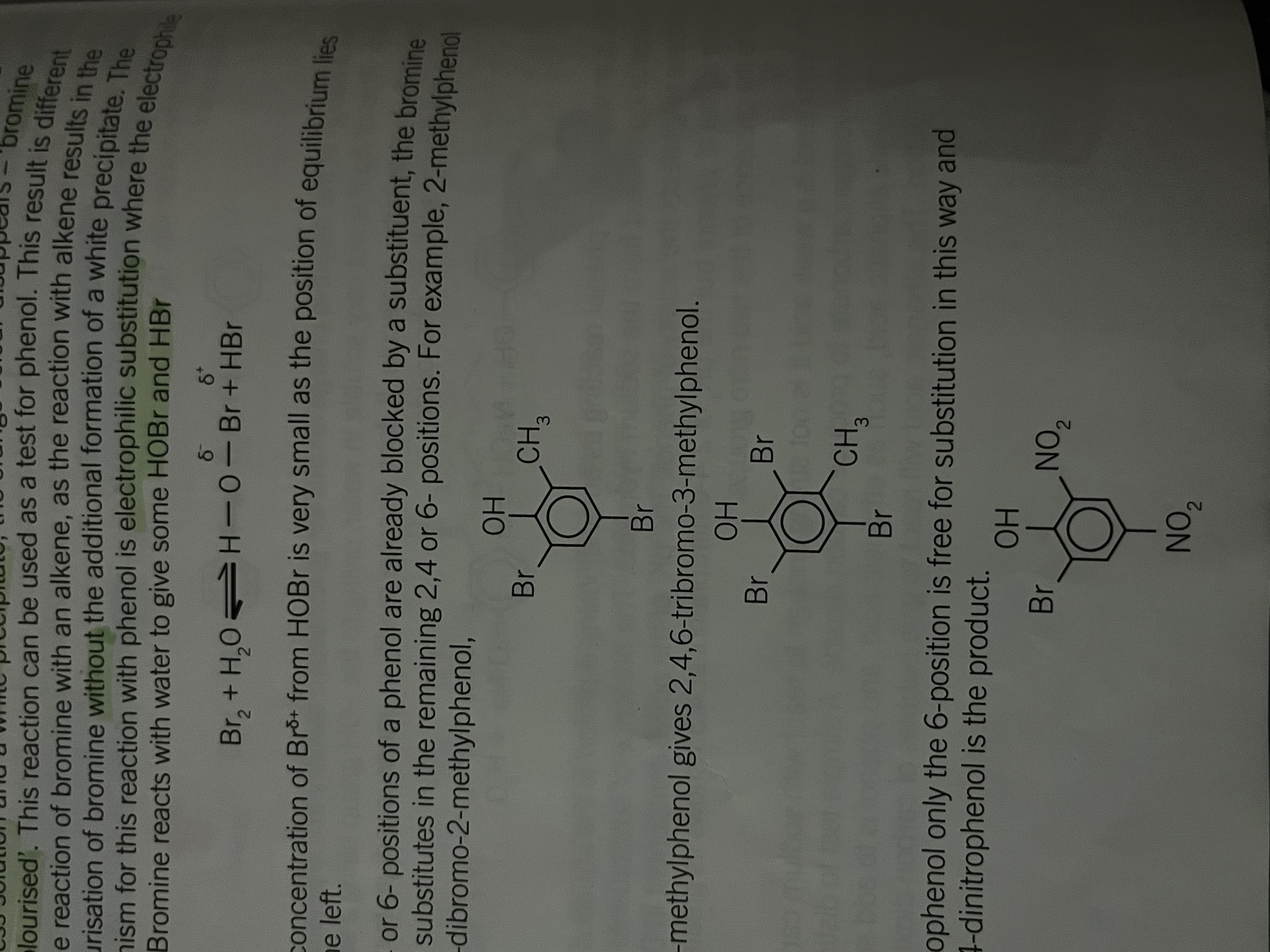
Directing effect of the -OH group
Has more activating effect on some positions around the ring than others
incoming groups will go into these positions much faster
has a 2,4,6 directing effect, incoming groups will tend to go into the 2, 4 or 6 position
Phenol with bromine
presence activate
Presence of an OH group bonded directly to a benzene ring will activate the ring to attack by electrophiles causing directing effect
when female reacts with bromine the increased electron density in the ring polarises the bromine molecules
Aqueous bromine reacts with phenol to produce a white precipitate of 2,4,6- tribromophenol
Colour change in the bromine test
Aqueous bromine= orange
products= colourless solution and a white precipitate
this means the orange colour disappears since the bromine is decolorized
How is reaction with bromine with phenol different to an alkene
In an alkene reaction, it will result in the decolorization of bromine without the additional formation of a white precipitate
mechanism for this reaction is electrophilic substitution. Electrophile is Br+
Bromine reaction with water
gives HOBr and HBr
Br2 + H2O = H-O-Br + HBr
concentration of Br+ from HOBr is very small as a position of the equilibrium lies well to the left
Reaction of Phenols and ethanoyl chloride
react as
Alcohols and phenols can react as nucleophiles by the use of their oxygen ion pairs
the delocalisation of an electron pair from the oxygen atom in a phenol means it is more difficult for a phenol to react as a nucleophile
as a result carboxylic acids are not suitable reagents to make an ester with phenol
Phenol and ethanoyl chloride
Slow at room temp
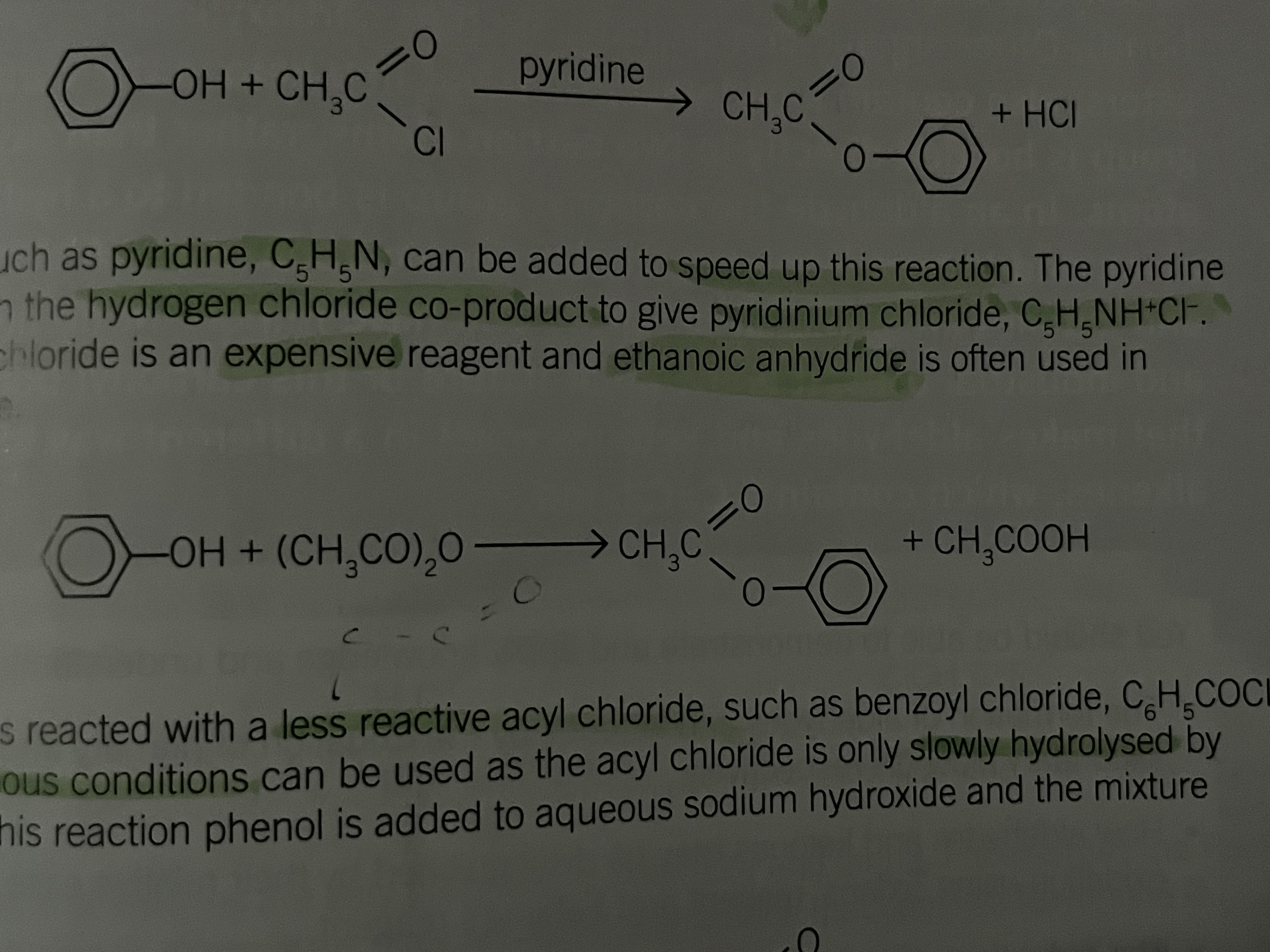
Phenol and a pyridine ( a base)
Can be added to speed up the reaction
the pyrdine react with the hydrogen chloride co-product to give pyridinium chloride (C5H5NH+Cl-)
Phenol and ethanoic anhydride
Ethanoyl chloride is an expensive reagent and ethanoic anhydride is used in preference
Phenol and acyl chloride
Less reactive
aqueous conditions can be used as the acyl chloride is only slowly hydrolysed by water
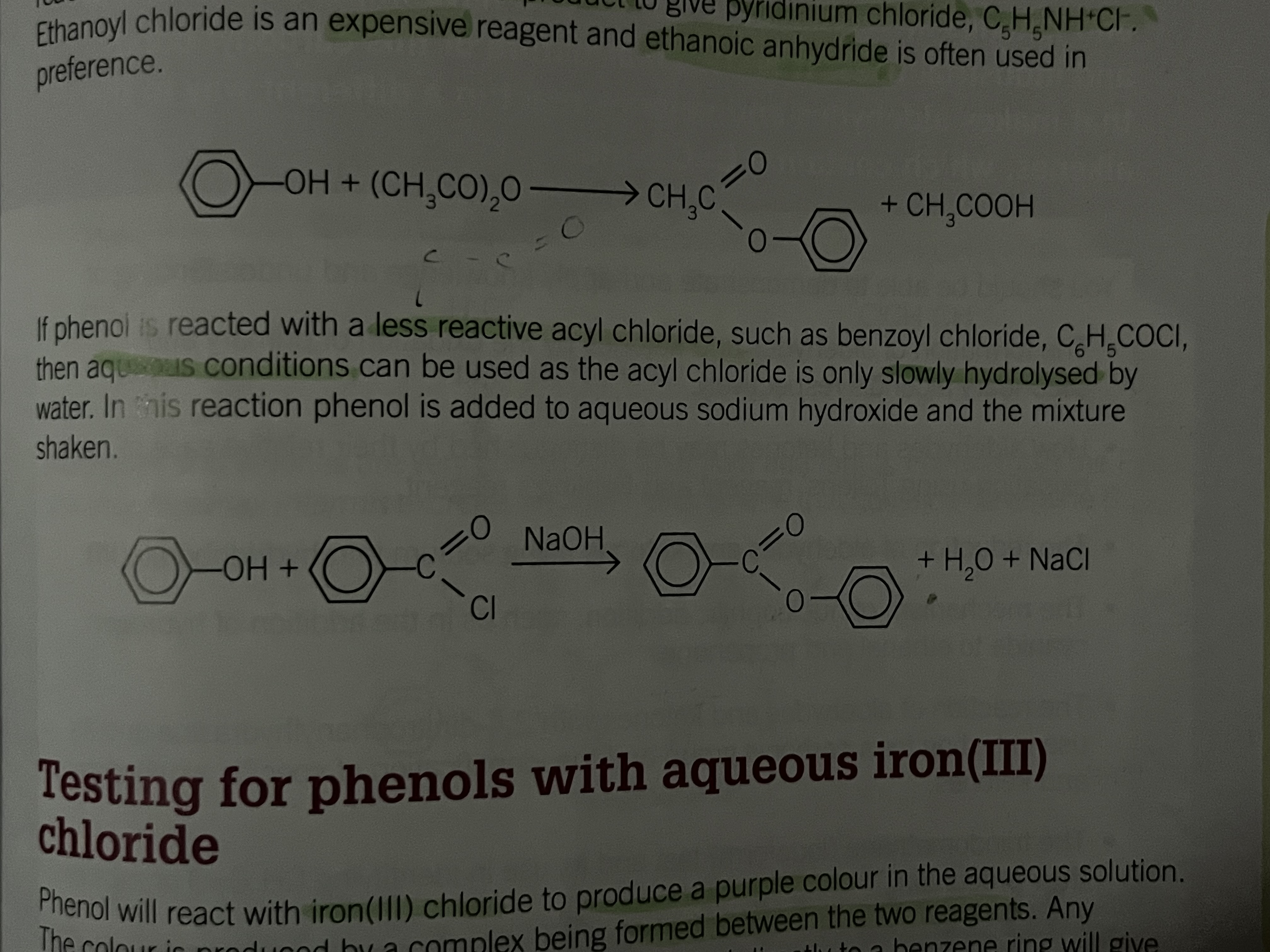
Test for phenols
Phenol will react with iron (lll) chloride to produce a purple color in the aqueous solution
the colour is produced by a complex being formed between the two reagents