Chapter 10 chemistry: The Mole
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
the relative scale of atomic masses in amu is also a relative scale of..
masses in grams
the atomic mass of an element, expressed in grams, in the…
the mass of 1 mole of that element
molecular mass is measured in
g/mol
Avogadro’s hypothesis
volumes of all gases at the same temperature and pressure contain equal numbers of particles
gases are compressible so under pressure particles are…
closer together
standard temperature and pressure (STP)
0°C and 1 ATM of pressure
molar volume of a gas is
the volume of 1 mole of the gas at a given temperature and pressure
At STP, 1 mole equals..
22.4L
Gas density
D= M/V
Volume fro any gas is the same
Density will then depend of molar mass
Measured in g/L
Percent composition
the percent by mass of each element in a compound
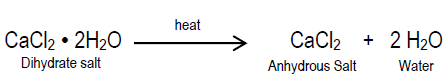
hydrate
a compound that has one or more water molecules bound to each formula unit
how to write a hydrate formula
the chemical formula, then a dot, and then the coefficient of how many water molecules there are per formula unit
empirical formula are written as
C₂H₄