Changing Subatomic Particles 1
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
What happens when the number of protons in an atom changes?
Changing the number of protons changes the element. For example, adding a proton to carbon (6 protons) makes it nitrogen (7 protons).
2
New cards
What happens when the number of neutrons in an atom changes?
Changing the number of neutrons creates an isotope, a variation of the same element with a different mass number.
3
New cards
What are isotopes?
are atoms of the same element with the same number of protons but different numbers of neutrons, resulting in different mass numbers.
4
New cards
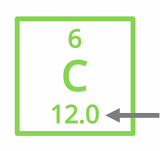
How is the atomic mass of an element calculated?
The atomic mass is a weighted average of an element's naturally occurring isotopes, considering their relative abundances.