Topic 3 - Polymers
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Define polymer
Long chain organic macromolecules
Macromolecules formed by the chemical bonding of large numbers of smaller molecules
Define monomer
A molecule which each can be linked to other monomers to form polymer chain
What are some naturally occuring polymers?
Hair
DNA
Spider silk
How are synthetic polymers produced?
They are produced by a polymerization reaction
What happens during polymerisation
C=C breaks apart
2 free electrons from the broken double bond combine with 2 other ethene molecules to form a chain
As it progresses, more and more ethene molecules are added to the chain resulting in a macro-molecule
Define polymeric materials
combination of carbon with oxygen, hydrogen, nitrogen and other organic/ inorganic elements.
Easily shaped into forms under heat and pressure
Define plastic and the two main types of plastic
Plastic: Large and varied group of synthetic materials formed or molded into shape
Thermoplastic: Polymer that can be melted
Thermoset: Cross-linked polymer that cannot be melted [silicone,, phenolics]
Define elastomers
Lightly crossed-linked macromolecules with rubbery viscoelastic properties [extremely flexible] [eg: Butyl, natural rubber, EPDM, neoprene, nitrile]
Thermoplastic
Soften when heated and harden when cooled [Reversible]
Melt and solidify without chemical changes and significant loss in mechanical properties
FOA between polymer chains can be overcome by heating thereby allowing the chain to slide over one another
Most linear polymers and some branched polymers are thermoplastics
Support hot-forming methods such as injection-moulding and FDM
Poor resistance to high temperature
Low strength but ductile
Thermosets
Harden the first time they are heated, do not soften after subsequent heating
During the initial heat treatment, covalent linkages are formed between chains [chains become cross-linked]
Do not melt with heating but will degrade at high enough temperatures
Networked/crosslinked polymers are typically thermosets
High thermal stability
Strong, rigid, hard, somewhat brittle
Name a few examples of thermoplastics
Polyethene (PE)
Polypropene(PP)
Polyvinyl chloride (PVC)
Polymethyl methacrylate (PMMA)
Polystyrene (PS)
Polyamide (PA-nylon)
Name a few examples of thermoset plastic
Epoxy
Silicone rubber
Polymelamin
Polyurethanes
What is a homopolymer?
polymer formed from one monomer [All repeating units are the same]
What is a copolymer?
Polymer made of 2 or more monomers
What are the 4 main structures of polymers?
Linear polymers [HDPE, PVC, Nylon]
Branched polymers [LDPE]
Crosslinked polymers [Rubber]
Network polymers [Kevlar, Epoxy]
![<ol><li><p>Linear polymers [HDPE, PVC, Nylon]</p></li><li><p>Branched polymers [LDPE]</p></li><li><p>Crosslinked polymers [Rubber]</p></li><li><p>Network polymers [Kevlar, Epoxy]</p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/a0996c91-70a5-4ae1-aa41-4908b765c592.png)
Describe the layout of an amorphous structure
No orderly arrangement of molecular chain in the solid state
When heated, shows no definite melting temp, but progressively becomes less rigid
Describe the layout of a crystalline structure
Ordered arrangement of the molecules in the solid state
distinct melting point, higher melting point
What are the effects of crystallinity on the mechanical properties and physical properties of a polymer?
The higher the crystallinity of polymer, the higher the melting point, the higher the tensile strength and modules of elasticity [resistance to deformation] of polymer
Define molecular weight
The mass of 1 mole of molecular chain [1 mole = 6.02×1023]
What are the effects of a higher molecular weight on properties of polymers?
Increases ductility
The higher degree of entanglement allows the material to be pulled further before the chains break
Increases the tensile & impact strength of the material
The higher degree of entanglement means that in order to rupture, more polymer bonds need to be broken, this means that the polymer can absorb more energy before failing
Increases the viscosity of the material
Harder to process the material using conventional methods. The longer the chains, the harder it is ti get them to flow because they are more tangled
Increases the chemical resistance
Takes more damage to the main chains of the molecules before it will affect the strength of the material
Explain what is glass transition temperature [Tg]
When an amorphous polymer is heated, the temperature at which it changes from a glass to the rubbery form is called Tg. This is the temp below which an amorphous soild (such as glass, polymers, tire rubber or cotton candy) goes from being ductile to brittle
Explain what is melting temperature [Tm]
For Crystalline or Semi-Crystalline materials, the melting temperature (Tm) is the temperature at which the crystals melt. Amorphous materials don’t truly have a Tm. They just continue to soften more until they behave more like a liquid. When we refer to the melt temperature for amorphous materials, it is usually the temperature at which we can process it.
What are the additives added to polymers to change the mechanical, chemical and physical properties?
Filler
Increases bulk and reduce cost
improve properties such as heat and chemical resistance
Plasticiser
Lowers Tg to improve ductility and softness
Stabiliser
Prevent degradation by heat and light
Colarant
Organic dye or inorganic pigment to form color
Lubricant
Reduce friction during processing or prevent sticking to molds
Flame retardant
Reduces the flammability of plastic
State the properties and applications of polyethylene
Properties:
Cheap
tough
flexible
Good chemical resistance
Applications:
LDPE: Bread bags, frozen food bags, grocery bags
HDPE: Milk, water and juice containers, toys, liquid detergent bottles
State the properties and applications of polypropylene
Properties:
Strength similar to HDPE, but easier to injection mold
Good fatigue properties
Excellent chemical resistance
Applications:
Gasoline tanks, chemical tanks, luggage, battery cases, ropes, fibers or filaments
Consumer products: Ketchup bottles, yogurt containers and margarine tubs, medicine bottles
State the properties and applications of Polyvinyl Chloride
Plasticised — low strength
Applications: decorative coatings, wire coating, imitation leather
Rigid — much stronger, tough resistance to grease/oil, resistance to chemicals, clarity, low cost
Applications: Chemical storage tank, piping, ducting
State the properties and application of polystyrene
Properties:
Brittle transparent material
Excellent electrical insulator
Application:
toy boxes, casing, radios
State the properties and application of polyamide
Properties:
strong and tough
relatively high melting point
low friction
Applications:
Gears, bearings, toothbrush bristles
State the properties and application of acrylonitrile-butadiene-styrene
Properties:
Tough, stiff, and abrasion resistance
Moderate rensile and compressive strengths
Remarkable dimensional stability
Applications:
Instrument panels
safety helmets
State the properties and application of acrylics
Properties:
Completely transparent themoplastics
Stiff strong with outstanding weather resistance
Applications:
Lenses for car lights, signs and nameplates
State the properties and application of Alkyds
Properties:
Hard and stiff
Good mechanical and electrical properties, dimensional stability
Applications:
Encapsulation of small electronic parts
State the properties and application of Phenolics
Properties:
High stiffness, impact, and heat resistance
Applications:
electrical plugs, sockets, switches
State the properties and application of Silicones
Properties:
excellent chemical inertness
low toxicity
Applications:
Water repellent properties
What are the differences between the processing of thermoplastic and thermoset?
Thermoplastic:
Raw polymeric materials are often in the form of pellets, granules, flakes or powders
Easily remelted and formed to the desired shapes
Examples: Injection Moulding, plastic extrusion
Thermoset:
Permanently set, with heat and catalysts, upon polymerization
Reheating will not change their shape aand heating at high temp will only cause disintegration and damage to the polymer
Raw material form monomer solutions or rod and sheet stocks
Examples: Compression Moulding, Transfer moulding
How does an injection mould work?
Polymer granules feed via hopper, polymer melts into molten state using heater bands and friction action of a reciprocating screw barrel
Heated plastic fills barrel, motorised screw pushes molten polymer into a mould
Injects molten polymer at high pressure into mould via gates and hot runners
The plastic product is allowed to cool and solidified before being ejected from the opened mould.
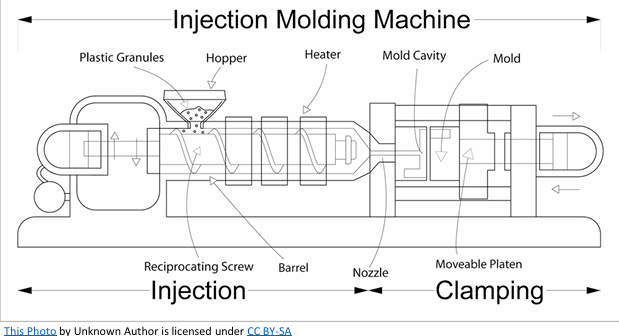
What are the advantages and disadvantages of injection moulding?

Explain the process of plastic extrusion
Plastic Extrusion Process is an operation for transforming a polymer using an extruder from the solid to the easily mouldable molten state, then discharging it through an extrusion die in a pre determined cross-sectional shape and re-solidifying by cooling.
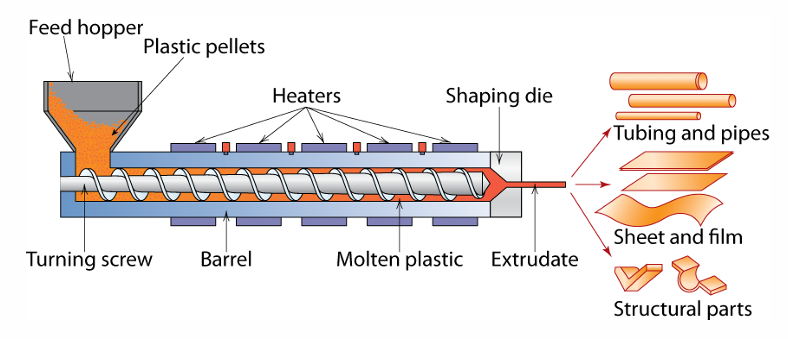
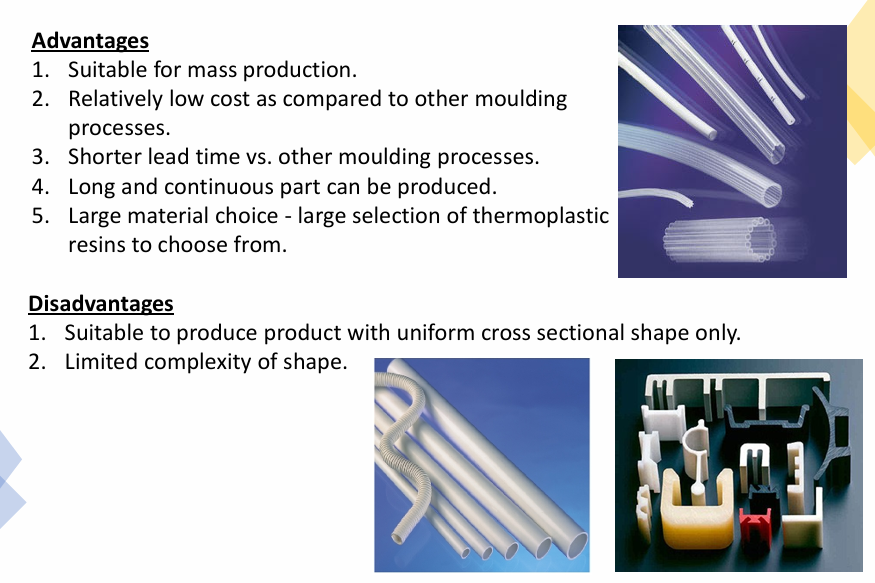
What are the advantages and disadvantages of plastic extrusion
Explain the process of compression moulding
Compression moulding is the simplest and most widely used process for making thermoset polymer products. Compound or blend is placed in mould and heated under pressure within the platens of a steam-heated press. When reaction is complete, product is cooled and ejected
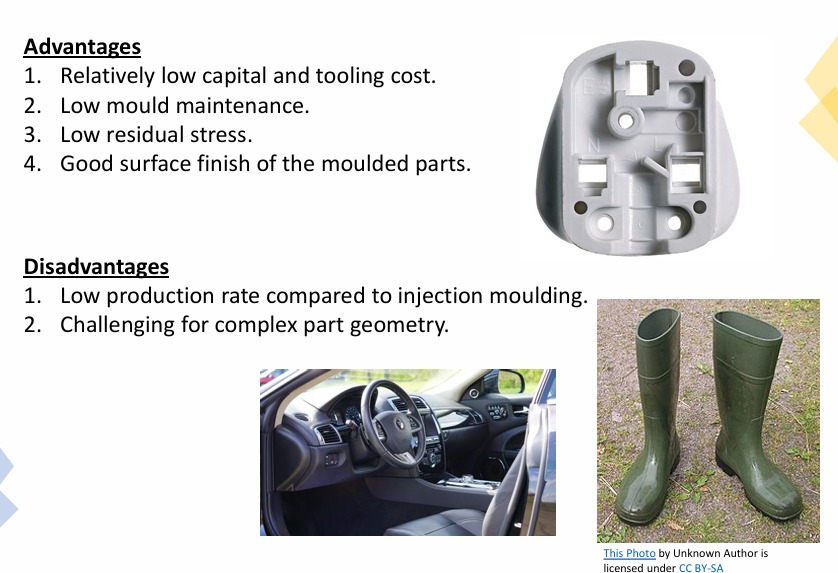
State the advantages and disadvantages of compression moulding
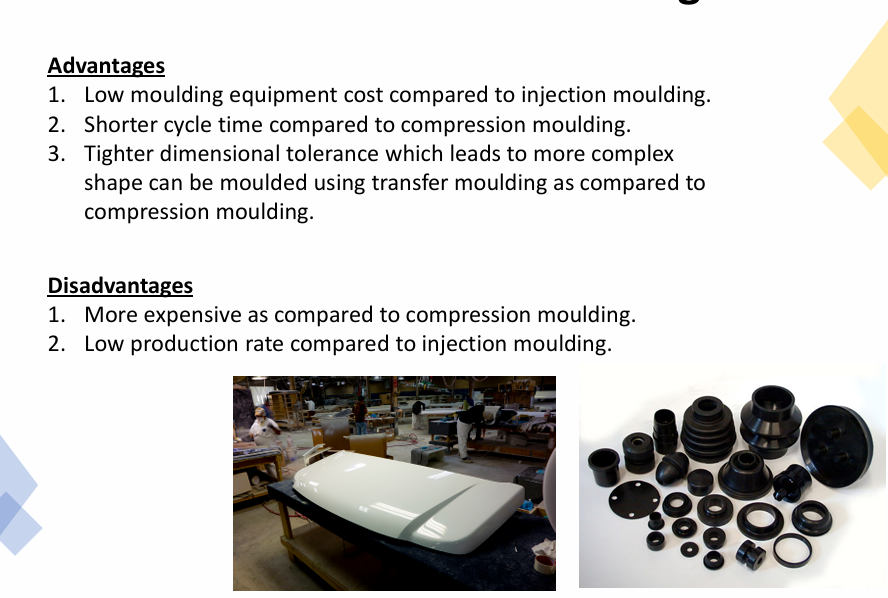
Explain the process of transfer moulding
Athermosetting charge (preform) is loaded and heated inside a chamber immediately ahead of the mould cavity
Pressure applied on the heated material using a plunger to force the softened polymer to flow into the heated mould
Pressure applied on the heated material using a plunger to force the softened polymer to flow into the heated mould
Athermosetting charge (preform) is loaded and heated inside a chamber immediately ahead of the mould cavity.
Pressure applied on the heated material using a plunger to force the softened polymer to flow into the heated mould.
The polymer within the mould cavities are permitted to cure before the final part is ejected from the mould.
State the advantages and disadvantages of transfer moulding