Block 4 - Pediatric Cancers
1/381
Earn XP
Description and Tags
ONCOL 309 - Clinical Oncology I. University of Alberta
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
382 Terms
Tumors in children tend to grow ______ and spread ____ than adult tumors
grow quicker and spread faster
Three most common types of pediatric cancers
Leukemia
Brain
CNS
What is the most common leukemia in children
Acute lymphoblastic leukemia (ALL)
What is the most common solid tumor in children
brain
Xeroderma Pigementosa
recessive gene unable to repair UV light damage
ex: p53 oncogene
Ataxia telangietasia
affects the cerebellum by causing poor coordination and weakens the immune system
ATM mutation
Bloom’s syndrome
a genetic disorder characterized by short stature, sun-sensitive skin changes, and increased cancer risk. It is caused by mutations in the BLM gene.
Fanconi’s Syndrome
a disorder in which the proximal tubule function of the kidney is impaired
Neurofibromatosis
nerve tissue grows tumor
Down’s Syndrome
extra copy of 21st chromosome
More than 2/3 of children can expect to be long term survivors of cancers due to what treatment application?
adding chemotherapy to surgery and RT
Radiation effects on pediatrics
Neuropsychological (CNS)
tissue hypoplasia
impaired growth
RT induced sarcomas
Chemotherapy effects on pediatric patients (3)
myocardial damage due to anthracyclines
nephrotoxcity due to cisplatin
secondary leukemias due to alkylating agents
how has the survival rates changed for pediatrics from the 1970s due the 2000s
1970s: 58%
2000s: 83.4%
how has the survival rate changed for ALL in pediatrics from 1975 to 2012
1975: 57%
2012: 92%
What is the prognosis for patients with glioma
poor; less than 1 year survival after diagnosis with no tumor improvemet
brain cancer is the leading cause of death among children
what is the primary treatment modality for pediatric cancers
surgery is the preference
RT and chemo are supplementary for surgery
what percent of children with cancer receive RT as part of their treatment
40-50%
what are the two goals of radiation therapy for pediatric patients
acheive tumor eradication
reduce treatment related toxicities
why is participation in clinical trials recommended for pediatric cancer cases
Participation in clinical trials is recommended for pediatric cancer cases because it provides access to new treatments and therapies that are not yet widely available, can lead to improvements in care, and contributes to the advancement of cancer research specifically tailored for children.
you will get the baseline treatment no matter what
Children’s Oncology Group (COG) leads clinical trial investigations for what percent of pediatric patients
50%
within the children’s oncology group (COG), there are special treatment considerations for trials under what four categories
data guidelines, concurrent chemo, immobilization, and anesthesia
5 challenges to radiation therapy treatment for pediatric patients
immobilization
anestheic or appropriate method
trust
allow pt to manipulte equipment
understanding
comprehension of RT is limitied
parents
protective of child, nervous and scared
medical team
multidisciplinary: lots of moving parts
what RT toxicities occur from treatment of pediatric patients to the epiphysis of bones
growth alteration
what is the RT dose threshold for effecting bone growth
10 Gy
there are some situations where 20 Gy or more are used
there is evidence of a dose reponse effect
there is a greater effect seen with a dose of >33 Gy than <33 Gy
what RT toxicities occur from treatment of pediatric patients from scatter to the gonads
affect development of sexual organs
increased risk of congenital deformity
RT side effects in boys after gondal radiation
germinal aplasia
gynecomastia
decreased testosterone
boys will have transient oligospermia after ____ but slow recovery can occur after _____
2 Gy, 2-5 Gy
For girls, oocytes are extremely senstive, but there has been reports of pregnancies after receiving _____ Gy
12 Gy
most times oocytes fail after 2 Gy
what RT toxicities occur from treatment of pediatric patients to the thyroid/pituitary
endocrine deficiencies
growth hormone after pit. irradiation
hypothyroidism
what RT toxicities occur from treatment of pediatric patients to the kidney
nephropathy
seens 2-3 years post RT (slow responding organ) with dose >15 Gy to both kidneys
what RT toxicities occur from treatment of pediatric patients to the lung
altered pulmonary function
what RT toxicities occur from treatment of pediatric patients to the brain
anatomic and functional abnormalities
decrease in cognitive capabilities
what RT toxicities occur from treatment of pediatric patients to the spinal cord
paresis and transverse myelitis
Lhermitte’s (electric shock-like sensation that travels down the spine and into the limbs upon forward flexion or movement of the neck) may result due to demyelination
what RT toxicities occur from treatment of pediatric patients to the liver
hepatomegaly, jaundice, ascites, thrombocytopenia, elevated transaminase
when are RT induced hepatopathy seen post RT?
usually occur 1-3 months post RT
Where do Ewing’s Sarcoma arise from
from primative cells in the body that form in the bone and soft tissue
What is a sarcoma
a tumor that arises out of connective tissues, most commonly found in the extremeties
can involve muscle and soft tissue around tumor site
EwS Epidemiology
Age range:
peak incidence:
M:F -
how many cases per 1 million
more common in what race:
childen aged 10-19
peak incidence at 15 years of age
M:F - 1.6:1
3 cases per 1 million
6x more common in caucasians vs. African americans
EwS is the second most common bone sarcoma in children behind ____
osteosarcoma
EwS Etiology
chromosome rearrangement found between what 3 chromosomes
chromosome aberration found in 95% of cases
rearrangement b/w chromosomes 11, 21, or 22
common locations of EwS
seen is diaphysis (shaft) of bones
53% in extremeties
47% in central axis
most common sites are femur and pelvis
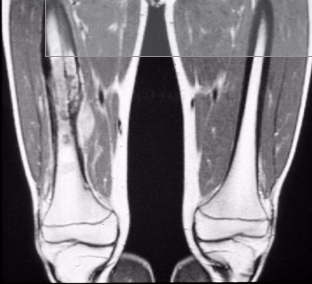
2 most common metastatic sites of EwS
lungs and bones
5 best prognostic treatment factors for EwS (pre-treatment)
distal or peripheral tumor
volume <200 ml
<14 years
female
no mets or mets in lung/bone only
5 worst prognostic treatment factors for EwS (pre-treatment)
tumor > 8 cm
axial tumor (spine sacrum)
14-20 years old
high WBC and fever
mets: lungs, multiple bones, bone marrow
3 post-treatment good prognostic indicators for EwS
female
younger age
minimal or no residual disease after pre-surgical therapy (chemo)
post-treatment poor prognostic indictors of EwS
poor response to pre-surgical chemo
increase risk of local recurrence
EwS natural history
occurs primarily in the bone but may also arise in soft tissue and muscle in close proximity to tumor
thought to arise from immature reticulum cells in the marrow cavity
local spread occurs along the marrow cavity causing bone destruction
what are the main sites of clinical presentation of EwS
predominantly in the diaphyisis (shaft) of these long bones
lower extremeties (41%)
pelvis (26%)
chest wall (16%)
upper extremeities
spine
skull (2%)
Early clinical presentation of EwS
swelling and pain near tumor site
90% complain of pain and 2/3 present with mass
warm lump in arms, legs, chest, pelvis
3 late systemic symptoms of EwS
fever
unexplained fracture
intratumor hemorrhage —> anemia, necrosis —> infection
What would a CBC and liver function test show in an EwS patient
CBC —> anemia if bone filtration
liver function test —> elevated LDH = advanced disease
what would a radiograph of the tumor site of EwS show
ill defined bone margins, soft tissue involvement
onion like appearance (lytic lesions)
moth eaten appearance
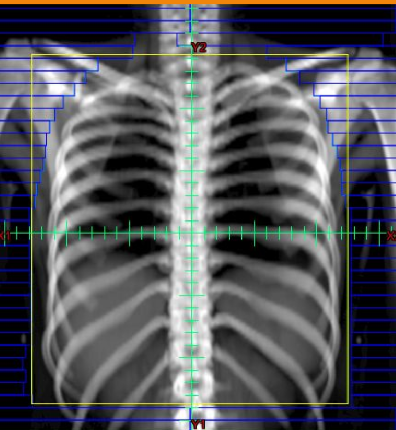
what 4 diagnostic imaging procedures can be done for EwS
CXR: check for mets
CT: bone destruction, lung mets, extent of tumor
MRI: soft tissue involvement
PET Scan: detect mets, intramedullary or blood spread
What 4 tumors are apart of the Ewing Sarcoma Family of tumors
Classic EwS
Primative neuroectodermal tumor
Askin tumor (of the chest wall)
Extraosseous Ewings Sarcoma
all have mesenchymal bone marrow stem cell origin
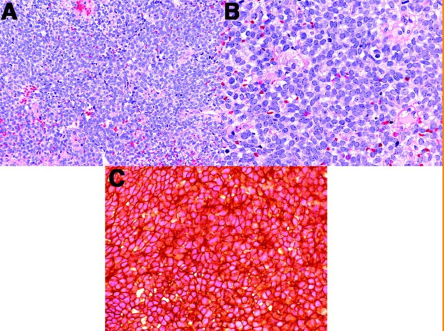
Histological features of EwS
round small blue cells with scant cytoplasm, high nuclear-to-cytoplasmic ratio, and usually associated with a fibrillary background.
three routes of spread for EwS
Hematogenous spread
Lymphatic spread
Direct invasion of adjacent tissues
What are the two stages of EwS
localized
clinial and imaging techniques, no spread beyond primary site, maybe some spread into adjacent of soft tissues
metastatic
Who is part of a treatment team in an EwS patient
surgeon
Med Onc
Radonc
peds nurse
socail work
rehab specialist
RT
why is chemo so important for ewings sarcoma
since most patients have occult (hidden) metastatic disease and EwS is very sensitive to chemotherapy
two treatment options for localized EwS
Neo-adjuvent chemo + surgery
RT + adjuvent chemotherapy
remember chemo is important to have
when may RT be used over surgery in EwS
when functionable and cosmetic morbidity or surgery is deemed to high
What is the multidrug approach for nearly all EwS cases
VAdriaC-IE
vincristine
doxorubicin (adriamycin)
cyclophosphamide
+ ifosamide and etoposide alternating every two weeks
duration of chemo for EwS
roughly 6 months
why is induction chemo important for EwS
Induction chemotherapy is crucial in Ewing Sarcoma (EwS) as it aims to reduce tumor size, control metastatic spread, and improve the overall response to subsequent treatments.
can also show how the tumor and patient responds to tumor
when is Stem cell support used alongside chemo for EwS
when patients have a high risk of relapse and a poor response to initial treatment
high dose treatment with autologous stem cell transplant may improve outcome
when is surgery recommended (although never primary over chemo)
when lesion is resectable
debulking procedure
total in expandable bone ( take all of rib/clavicle)
amputation
limb-salvage surgery is possible
What two reasons would RT be recommended for EwS
patients with incomplete resection after surgery
patients who would suffer loss of function after surgery
when is adjuvent RT used after surgery
residual microscopic disease
inadequate surgical margins
viable tumor in resected portion and close surgical margins
what energy is used for RT in EwS treatment
6 MV
these are children, this is more than enough
immobilization and position varies for age of patient and tumor site
dose / fractionation and margins for pt had pre-chemo and no Sx
50-60 Gy / 25-30 days
2 cm margins
EwS dose / fractionation and margins if pt had surgery (2 phases)
2 phases
4500 cGy/ 25 fraction to whole bones (minus growth plate)
1000 cGy / 5 fractions boost to tumor bed with generous margin
for high risk tumor area
what treatment technique is typically used for EwS patients
IMRT
why are the fields shaped to maintain a route for lymphatic drainage in long bones?
To prevent lymphedema and preserve function.
decreases QoL
What should RT fields for EwS patients include
affected limb and full length of surgical scar
want to include bolus
What is the shrinking field technique for EwS patients
the two phase boost approach previously mentioned
tumor + margin for microscopic disease and nodes
reduce size of field to include disease and minimize margin in second phase
exclude epiphyseal plate (marrow) this will effect delivery of chemo
why would electron RT make sense for cancers in the ribs
will reduce dose to lungs
what three factors does RT treatment of metastatic or recurrent EwS depend on
site of recurrence
prior treatment
individual consideration
EwS pulmonary mets RT plan
Energy
Technique
Dose
Margins
whole lung RT:
POP
6 MV
12-20 Gy to relieve cough and dyspnea
include apex of lung and 1 cm of lateral space
we dont treat brachial plexus and humoral head so we shield them
Acute skin side effects for EwS patients
erythema, dry and moist desquamation
Chronic skin side effects for EwS patients
telangietasia and hyperpigmentation
Side effects in muscle for EwS patients
atrophy
hypoplasia occurs after 20 Gy
physio required
Side effects in bone for Ews Patients
shortening and weakening
arrested bone development occurs after 20 Gy
what dose do we we see an increased risk of 2nd malignancies for EwS patients
60 Gy
Survival for localized EwS of the bone
70% at 5 years
survival for metastatic EwS of the bone
30% at 6 years
Survival for lung, bone, and both mets
lung: 40% at 4 years
bone: 28% at 4 years
Combined: 14% at 4 years
Future considerations for EwS treatment
better systemic agents
genetic therapy targeting translocations that cause malignancy
Bone and stem cell transplants
what is a wilm’s tumor
A childhood kidney cancer that usually occurs in children aged 3 to 4 years. It is characterized by the presence of a tumor on one or both kidneys and often presents with abdominal swelling or pain.
aka nephroblastoma
Wilm’s tumor epidemiology -
Peak incidence:
more common in which gender:
most common ___ cancer in children
what ethnicities is it most common in:
rare tumor
3-5 years
more common in females
most common abdominal cancer
more common in Africans
three associated GU abnomalities in children that result in Wilms tumor screening every three months until age 8
hemihypertrophy
cryptoorchidism
hypospadias
4 phenotypic syndromes of Wilm’s Tumor
Denys-Drash syndrome
90% risk
WAGR syndrome (wilms, anirida, genital abnormalities, retardation)
50% chance
Beckwith-Wiedemann Syndrome
10% risk
Mutations of WT1 gene at chromosome 11
Poor prognostic indicators of Wilm’s tumor (5)
stage of disease at diagnosis (worse than stage 1)
diffuse anaplasia
age of patient (worse >18 years old)
tumor size at presentation (large)
LN involvement
Early Clinical Presentation of Wilm’s tumor
patients typically present with an asymptomatic abdominal mass, but also may present with pain
in addition to: hematuria, UTI, fever, anemia
Late Clinical Presentation of Wilm’s tumor
pain at distant mets
internal bleeding from ruptured tumor
respiratory distress (abdominal distentions ± pulmonary mets)
How is Wilm’s tumor diagnosed
physical and history (past and family)
routine lab work: CBC, blood chemistry, liver renal function
What 3 imaging modalities can be used to diagnose Wilm’s tumor
abdominal U.S
Chest + Abdo X-Ray
CT - Chest/Abdo
what 4 imaging modalities can be used to stage Wilm’s
CT
X-ray
MRI
ultrasound
what are the two histologic groups for Wilm’s tumor
favourable
unfavourable (anaplastic)
Favourable Wilm’s pathology -
mimics ___ development
prognosis and chemo response
mimics normal kidney development
3 cell types (blastemal, epithelial, stromal)
blastemal = most malignant component
better prognosis
better chemo response