Chemical formulas
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
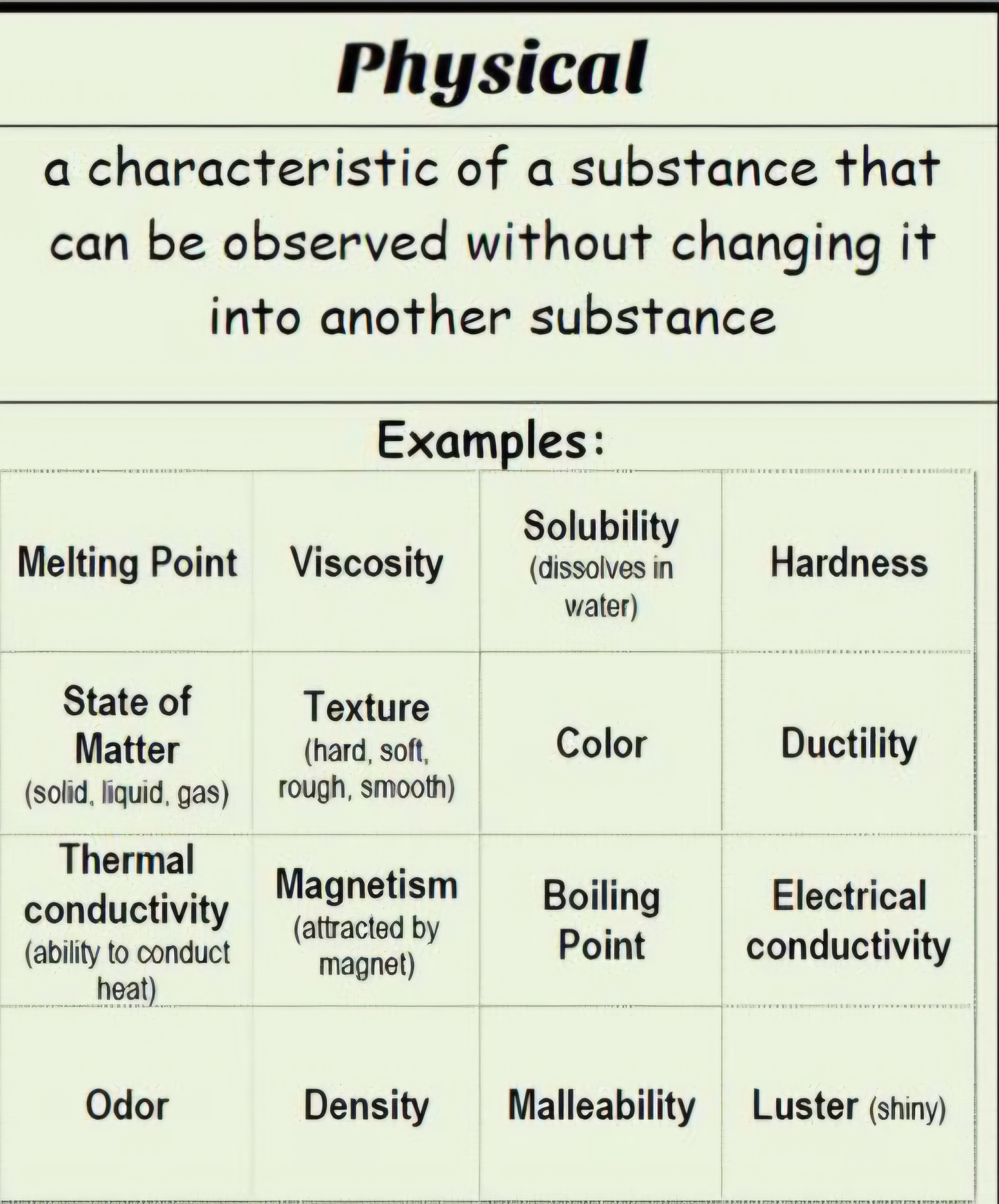
A physical poperty
A characteristic or trait that can be observed without changing the substance into something new
A Chemical property
A characteristic that describes the ability to change into a different substance. To observe the chemical properties of a substance, you must try to change it to another substance.


Chemical changes
Produces new compounds
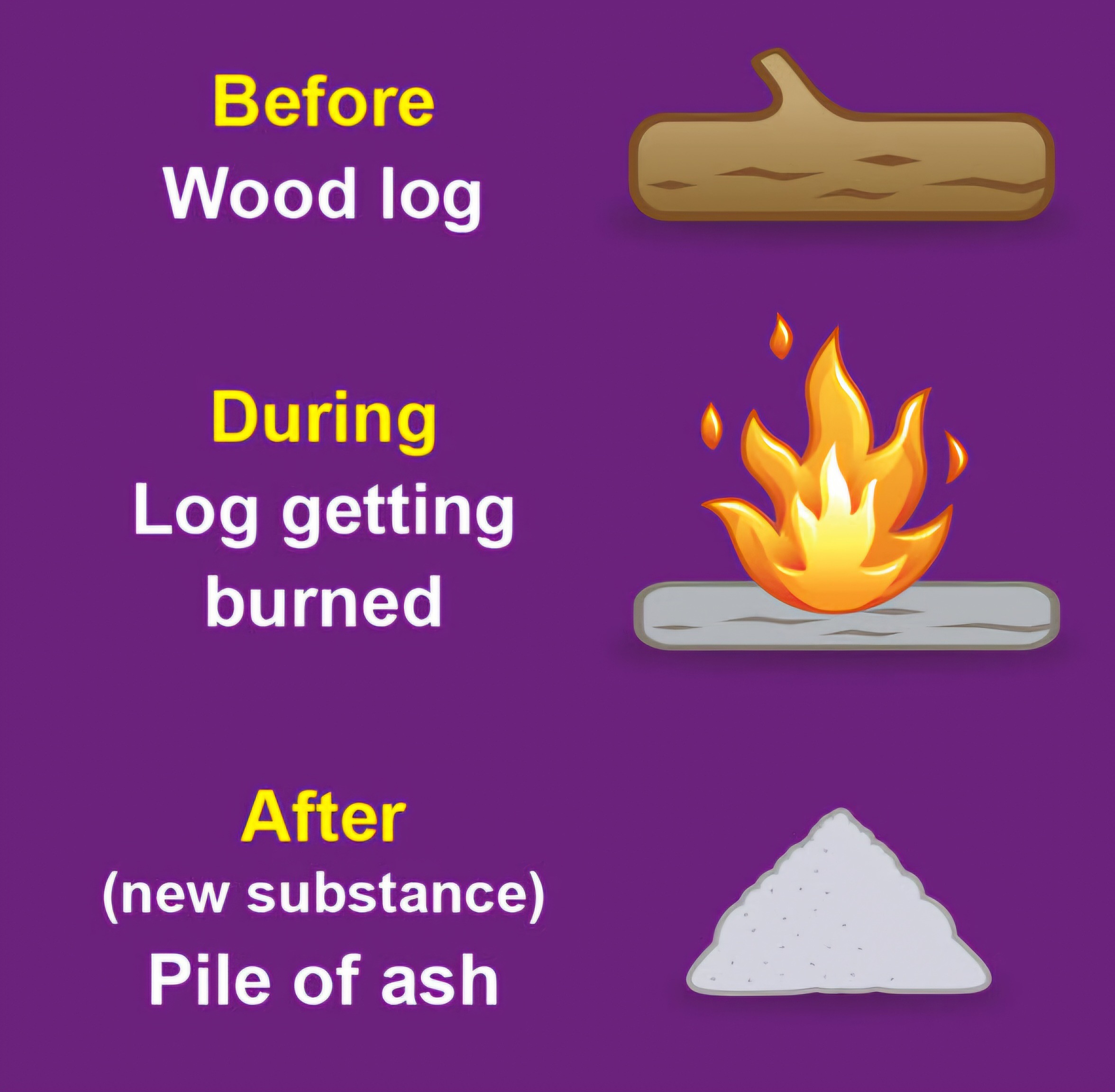

Physical change
Does not change the substance
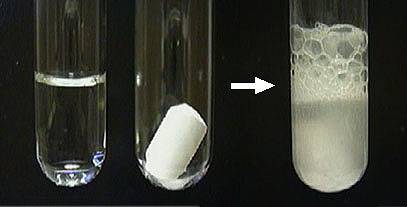
Production of gas is what kind of change
Chemical


Sand being washed out to sea is what kind of change
Physical


Production of energy (light, heat, sound, odor)
Chemical


Change in a substance form is a
Physical change


Production of precipitate (new solid) is what kind of change
Chemical


Breaking the substance is what kind of change
Physical

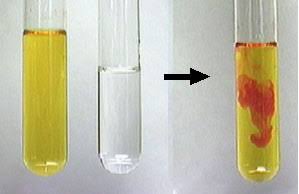
What kind of change is an unexpected color change
Chemical
What change is reversible
Physical


Melting is what kind of change
Physical

Evaporation is what kind of change
Physicsl

Acid rain damaging car paint
Chemical
Chemical formula, like H₂O, tells us what 2 things
The elements that are present and how many of them there are.
Atoms are stable if
Their valence level is full of electrons
What is an ion
An atom that is positively or negatively charged
When chemical changes produce new compounds do the new compounds have same properties of the original elements?
No
When an atom loses electrons what charge is the ion?
Positive
When an atom gains electrons what charge is the ion?
Negative
The number tells us how many electrons have been gained or lost is the
Oxidation number
Why do atoms form chemical bonds?
They want their valence levels to be full
When writing formulas, does the element with the positive or negative oxidation number come first?
Positive
Superscript shows us
The oxidation number
Covalent bonds are formed between
2 non-metals
Ionic bonds are formed between
1 metal and 1 nonmetal
Ionic bonds occur when
Elements give or take electrons
Covalant bonds happen when
Elements share electrons
Polyatomic ions must
Stay together in a chemical formula and are covalently bonded.
Subscript tells us
How many atoms of that element are present
Why is Magnesium (II) oxide the wrong name
Roman numerals in chemical names are used to indicate the oxidation state. Magnesium does not have a variable oxidation state.
Name this compound
KClO₃
Potassium Chlorate
The number that tells us how many electrons have been gained or last is known as
The oxidation number
Name this compound
CuCl₂
Copper (II) Sulfate
Sodium Carbonate oxidation numbers are
Na⁺ CO₃²⁻ so it’s formula is
Na₂CO₃
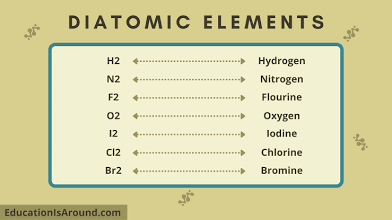
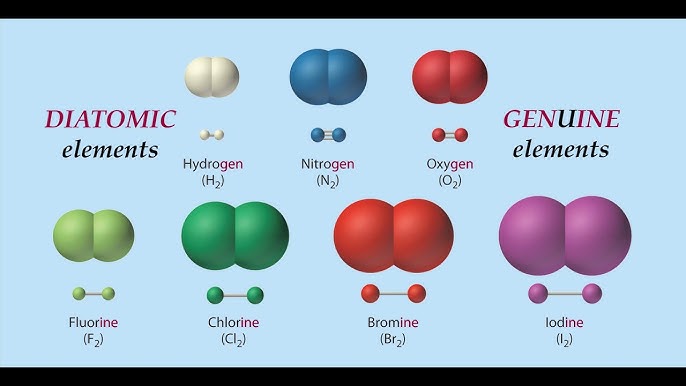
Diatomic molecules
A molecule composed of two atoms of the same element
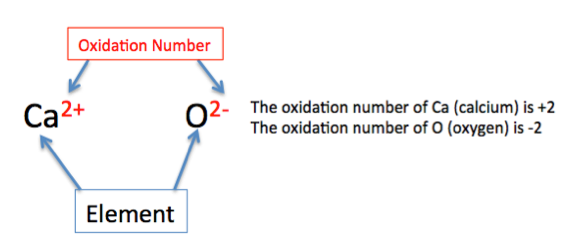
Are elements with a positive oxidation numbers written first in the chemical formula?
Yes
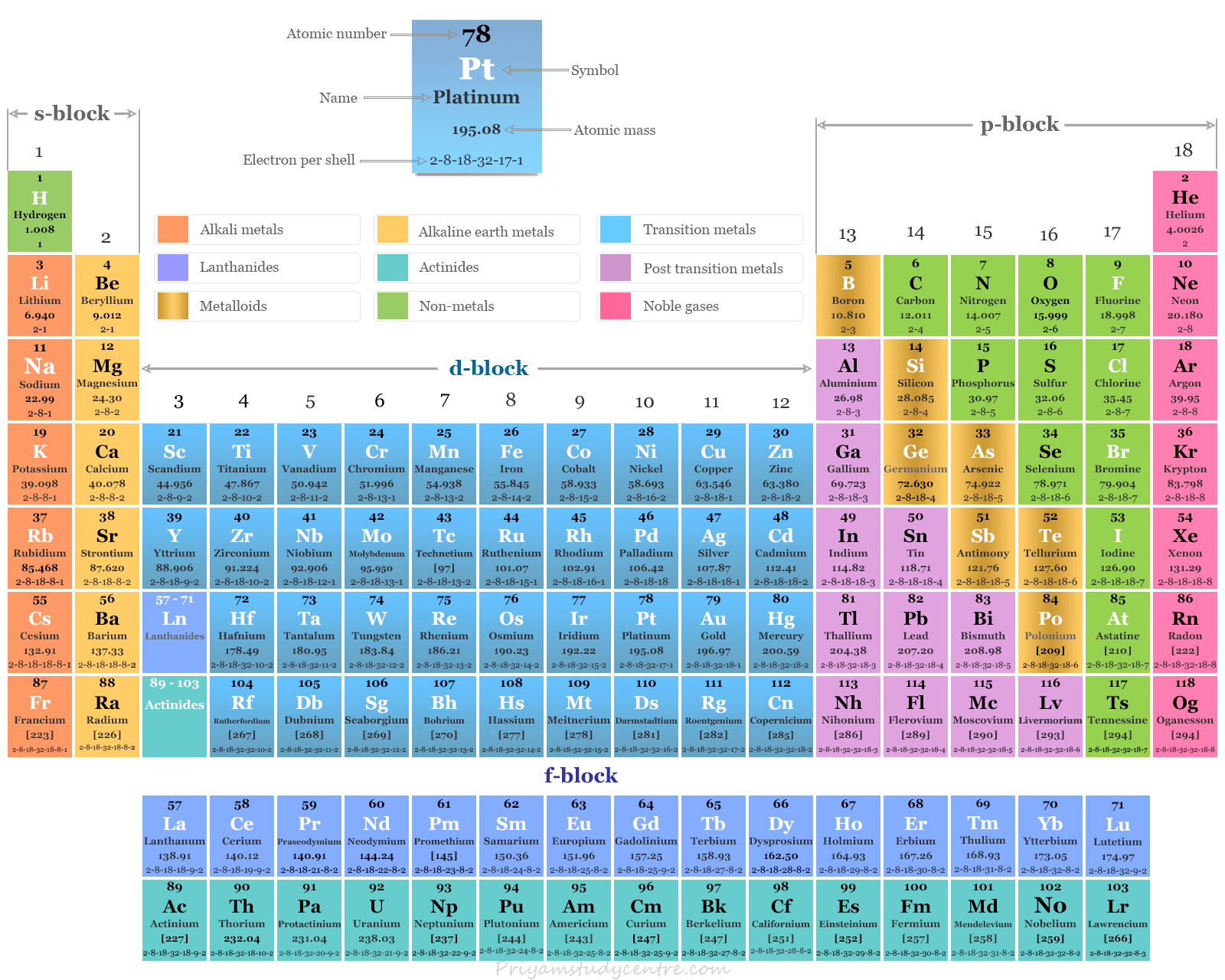
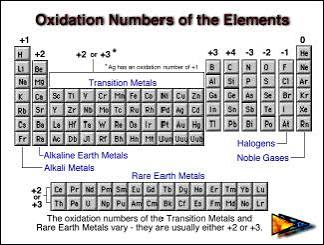
Where on the periodic table are the elements with a variable oxidation number
Middle, center or are the transition metals
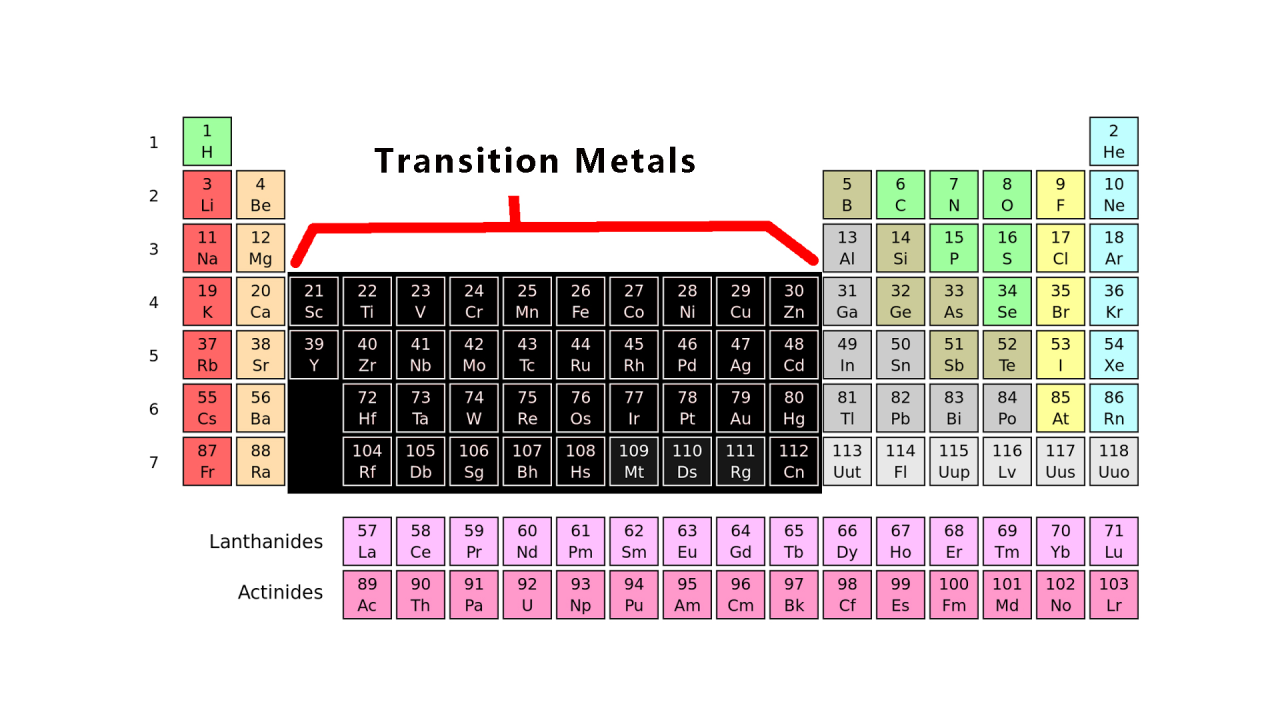
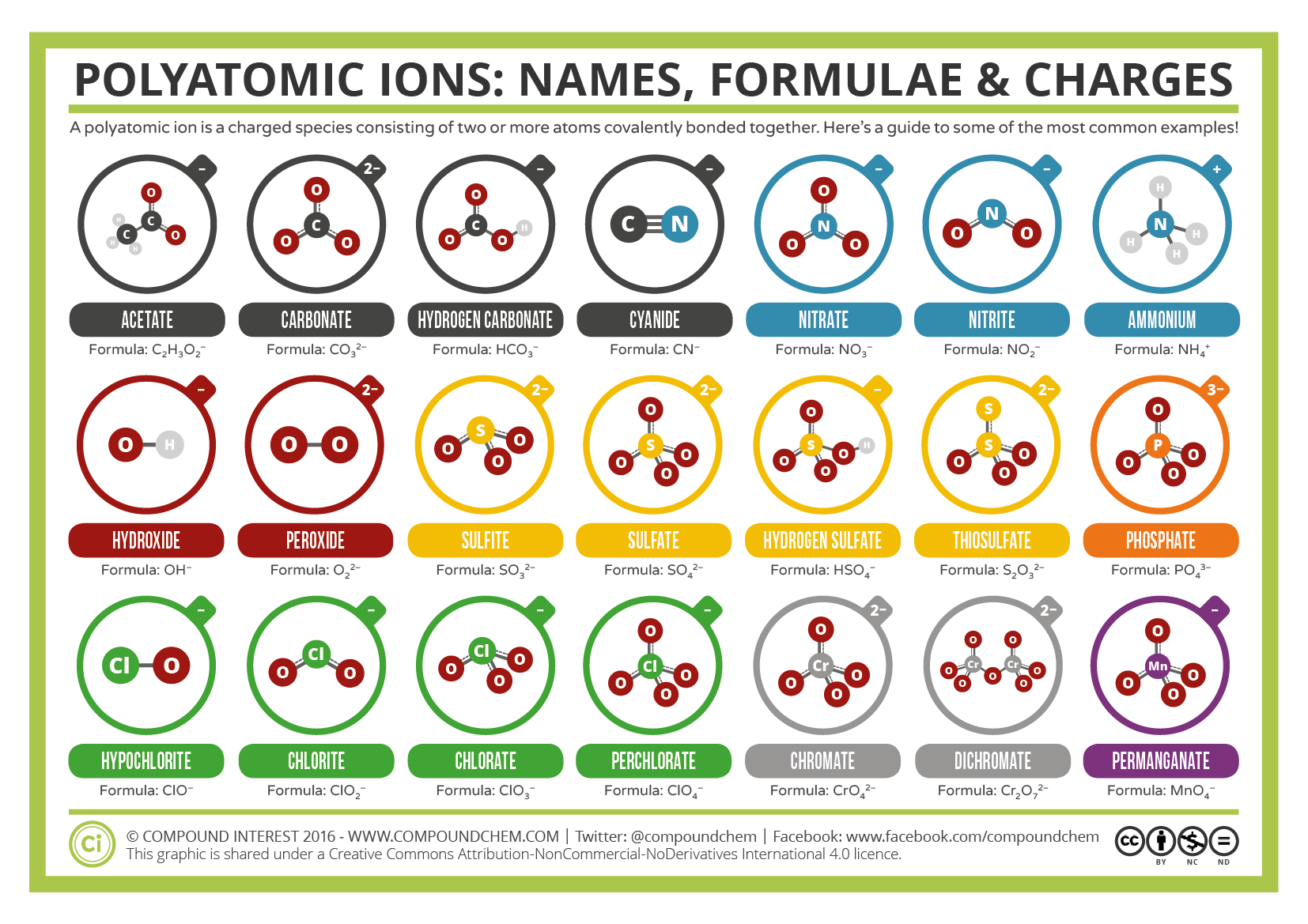
What is a polyatomic ion?
A group of atoms that react as one unit and have an overall positive or negative charge.
Examples: ammonium NH4+
(is a group of 1Nitrogen and 4Hydrogens)
nitrite NO2−
(Group of 1Nitrogen 2 Oxygen)
hydroxide OH−
(group of 1 Oxygen and 1Hydrogen)
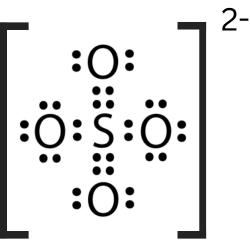

Two clear liquids are mixed and a yellow color with chucks forms. What evidence is there that a chemical reaction has formed?
The appearance of a yellow color and a precipitate upon mixing two clear liquids indicates a chemical change, as new substances are formed through a chemical reaction.
