crude oil part 1
1/24
Earn XP
Description and Tags
yes
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
hydrocarbon
a molecule that is only made from hydrogen and carbon atoms
crude oil formation
Crude oil forms over millions of years from tiny dead sea creatures buried under layers of mud. Heat and pressure slowly turn them into oil, which then moves through rocks until trapped underground.
what are they alkanes
simplest type of hydrocarbons with only single bonds
saturated hydrocarbons as they have as many hydrogen atoms as possible
alkane formula
CnH2n+2
properties of hydrocarbons
flammability (decreases with bigger molecule size )
viscosity (increases with bigger molecule size)
boiling point(increases with bigger molecule size)
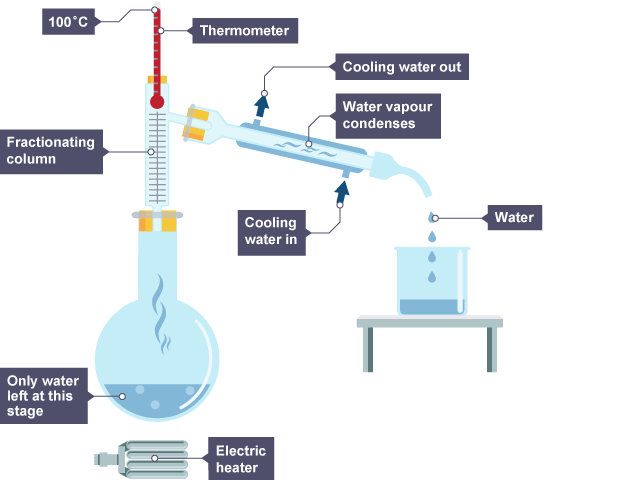
what is fractional dislitation
a method of seperating two liquids from a liquid, for example ink and water. ink has a higher boiling point than water so water would evaporate first which seperates them
complete combustion
complete combustion of a hydrocarbon produces water vapour and carbon dioxidec
incomplete combustion
Incomplete combustion. happens when the supply of air or oxygen is poor
example of fractional distilation
cracking hydrocarbons
when you break c-c bonds in a hydrocarbon
2 typer of hydrocarbon cracking
thermal, using high temperature and pressure
catalytic, using a catalyst to speed up the process using low temps
alkenes
hydrocarbons with double bonds (c=c)
unsaturated hydrocarbons because not have max nom of hydrogen atoms
alkene equation
CnH2n
test for alkanes and alkenes
bromine water test
bromine water test result for alkenes
Alkenes – Turn orange bromine water colorless (because they react with bromine in an addition reaction).
bromine water test result for alkanes
Alkanes – No color change, bromine water stays orange (because alkanes do not react with bromine under normal conditions).
how does the bromine water test work
This test works because alkenes have a double bond, allowing them to react with bromine, while alkanes only have single bonds and are unreactive.