CHROMATOGRAPHY
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
74 Terms
Chromatography
A separation technique for gases, liquids, and dissolved substances based on the different degrees of attractive interaction of molecules to either the mobile phase or the stationary phase
affinity
Any kind of chromatography will separate substances based on their _________ with the mobile phase and the stationary phase.
Stationary Phase (SP)
A phase in chromatography that is a layer or coating on the supporting medium (column or planar surface) which interacts with the analytes.
silica, alumina, and C-18 (for paper chromatography)
TLC, column chromatography, and paper chromatography contain adsorbents such as?
Mobile Phase (MP)
A phase in chromatography that is a part of the chromatographic system that carries the solute across the “other” phase. It is also referred to as the solvent systems/menstruum.
capillary action; upwards
gravity; downward
Thin layer chromotography (TLC) uses _______, hence the movement is ________
Column chromotography uses ________, hence the movemnt is ________
slower
Substances that interact more with the stationary phase are less soluble in the mobile phase, hence they travel ________
faster
Substances that interact more with the mobile phase are less soluble in the stationary phase, hence they travel ________
analyte
What do you call the substance that needs to be separated or identified from a mixture?
eluent
What do you call the mobile phase or the solvent or fluid that enters the column and carries the sample through the column?
eluate
What do you call the mixture that exits the column after the separation has occurred (contains both the separated sample and the eluent)?
first/faster
end
Column chromatography: If the analyte has a lower affinity (weaker adhesive interaction) with the stationary phase, the eluate comes out _____ or is found at the _____ of the column.
last/slower
top
Column chromatography: If the analyte has a higher affinity (stronger adhesive interaction) with the stationary phase, the eluate comes out _____ or is found at the _____ of the column.
faster
top
Thin Layer Chromatography: If the analyte has a lower affinity (weaker adhesive interaction) with the stationary phase, the eluate travels _____ or is found at the _____ of the plate.
slower
bottom
Thin Layer Chromatography: If the analyte has a higher affinity (stronger adhesive interaction) with the stationary phase, the eluate travels _____ or is found at the _____ of the plate.
faster/farther
If the mobile phase is polar, the polar compounds dissolve better in it and they move ________
faster/farther
If the mobile phase is nonpolar, the nonpolar compounds dissolve better in it and they move ________
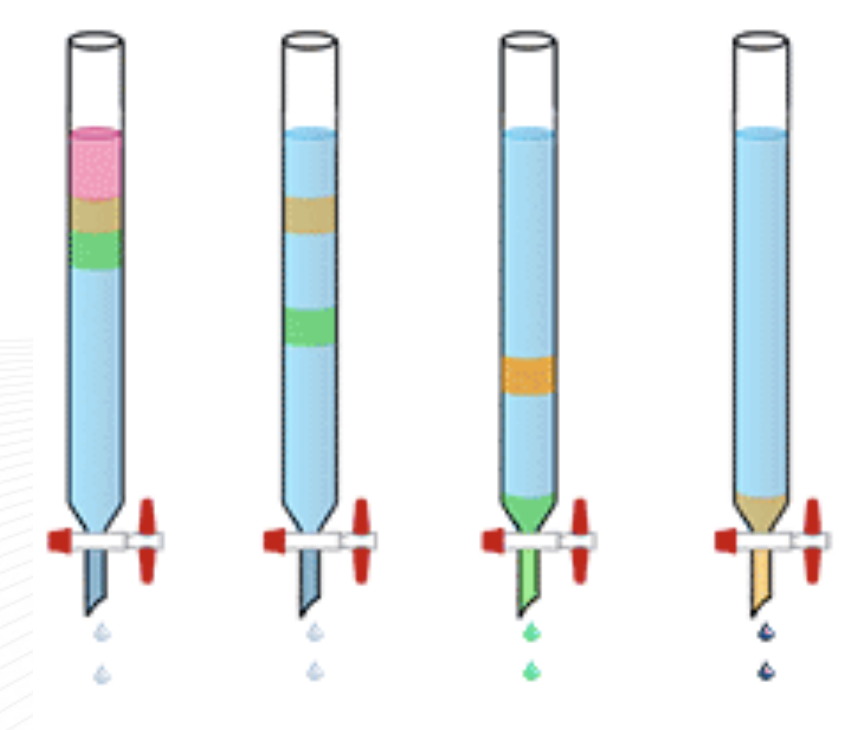
orange (migrates last)
the sample is polar
Silica gel is a polar stationary phase in column chromatography. If the sample is highly adherent to the silica gel, what color is that sample in the diagram? Is the sample polar/nonpolar?
Polarity
Hydrophobicity/Non-polarity
Ionic interactions
Hydrogen bonding
Particle size
Structural complementarity
Chromatography relies on the what properties of the substance?
Polar (e.g., silica)
Nonpolar (e.g., chloroform-methanol, 3:1)
Based on the nature of MP and SP: If the nature is Normal
Stationary phase: _________
Mobile phase: _________
Nonpolar (e.g., ODS/octadecylsilane, C-18)
Polar (e.g., ACN/acetonitrile, Methanol)
Based on the nature of MP and SP: If the nature is Reverse
Stationary phase: _________
Mobile phase: _________
capillary action (e.g., TLC, paper chromatography)
gravitational pull (e.g., column chromatography)
Based on the direction of movement of the solvent:
Ascending: Aided by the ________ of the solvent
Descending: Aided by the ________ to the solvent
isocratic; constant
gradient; gradual change
Based on concentration of solvent:
_______: one (1) mobile phase is used. It involves a _______ composition (solvent strength) of the mobile phase used. Longer time needed.
_______: more than or equal to two (2) mobile phases are used. It involves a _______ in the mobile phase composition
Partition; solubility (e.g., paper chromatography)
Adsorption; adsorption (e.g., silica gel chromatography, TLC)
Based on the Mechanism of Separation:
Particles are separated into the components of the solvent system. It is the distribution between two immiscible liquids. It is based on the ______ of different components
Particles are adsorbed on the solid support. It is based on the differential ______ of components to the stationary phase
Either Normal (common) or Reverse (Nature of MP and SP)
Ascending
Isocratic
Adsorption
Classification of Thin Layer Chromatography (TLC):
Based on the nature of MP and SP: _____
Based on the direction of movement of the solvent: _____
Based on concentration of solvent: _____
Based on the Mechanism of Separation: _____
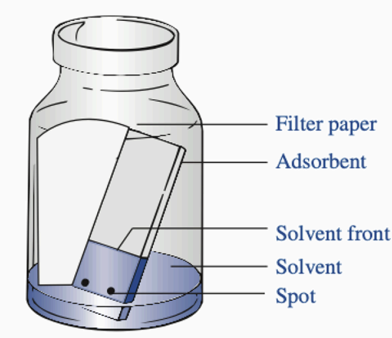
Filter paper
Parts of TLC: Used to saturate the atmosphere with solvent vapors. Preventing the drying of the upper portions of the stationary phase.
Adsorbent
Parts of TLC: Acts as a stationary phase, a thin layer of silica gel or alumina on a plate
Solvent Front
Parts of TLC: The highest point that the solvent reaches on the TLC plate
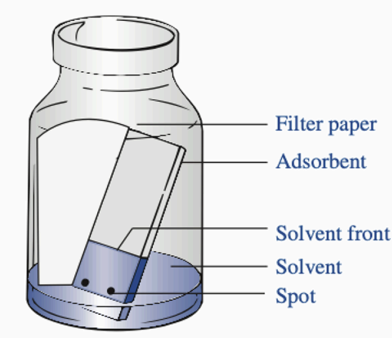
Solvent
Parts of TLC: The mobile phase, which aids the flow of substances through the stationary phase
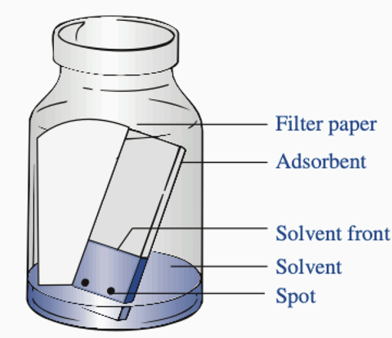
Spot
Parts of TLC: Contains the sample/substances to be differentiated or separated. Ideally, around 2mm in diameter
Retention factor (Rf)
A ratio that indicates how far the solute travels relative to the solvent front
less
faster/farther
top
Higher RF suggests that there is _____ interaction/affinity to the stationary phase, and thus will travel _____. The sample will most likely end up at the _____ of the plate.
far; mobile
close; stationary
High Rf = ____ migration = prefers _____ phase
Low Rf = ____ migration = prefers _____ phase
inversely
Retention factor (Rf) is _______ proportional to the affinity of the sample (for the stationary phase).
directly
Retention factor (Rf) is _______ proportional to the distance travelled by the sample.
Rf = distance travelled by solute/distance travelled by solvent front
Formula for Rf value
higher
TLC Selecting Stationary Phase: In Normal chromatography, the stationary phase is polar. So, the higher the polarity, the ______ the adsorption on polar stationary phases
lower
TLC Selecting Stationary Phase: In Reverse chromatography, the stationary phase is nonpolar. So, the higher the polarity, the ______ the adsorption on polar stationary phases.
nonpolar
less
Alkyl halides are _______ hence are ______ absorbed in polar stationary phases.
eluting power
The ability of a solvent (mobile) phase to transport compounds with it through the stationary phase.
highly
less
TLC Selecting Mobile Phase: Polar compounds are best eluted/transported with a ____ polar mobile phase, because it can shift the affinity of the compound to itself and poorly eluted/transported with a ____ polar mobile phase.
Recall that in a standard TLC setup, the stationary phase (the plate) is made of a polar material, most commonly silica gel.
polar
higher
TLC Selecting Mobile Phase: Nonpolar compounds are best eluted/transported when a ______ stationary phase is used. It is carried easily by the mobile phase, traveling a _____ distance up the plate.
less
close
TLC Selecting Mobile Phase: A polar compound will be poorly eluted with a ____ polar mobile phase. In this scenario, the polar compound will have a stronger affinity for the polar stationary phase than for the less polar mobile phase, causing it to remain ____ to the starting point.
higher
higher
lower
In TLC, the higher the eluting power,
the ________ the solvent strength'
the ________ the adsorption of polar mobile phase
the ________ the migration
Water, methanol, ethanol, propanol, and acetone are polar solvents, which means it has a high affinity to the polar SP. If a nonpolar sample would pass through it, it would travel close from the starting point as it is insoluble in the MP
Principle of “like dissolves like” in TLC: If water, methanol, ethanol, propanol, and acetone are the solvents used (MP), what will most likely happen to a nonpolar sample? Will it travel far/close from the sample spot (starting point)? Why?
Petroleum ether, hexane, toluene, and dichloromethane (DCM) are nonpolar solvents, which means it has a weak interaction with the stationary phase of TLC (silica is polar) and the nonpolar sample won't be held back, causing it to travel further up the plate.
Principle of “like dissolves like” in TLC: If petroleum ether, hexane, toluene, and dichloromethane (DCM) are the solvents used (MP), what will most likely happen to a nonpolar sample? Will it travel far/close from the sample spot (starting point)? Why?
Water, methanol, ethanol, propanol, and acetone are polar solvents, which means it has a high affinity to the polar SP. Since the SP (silica) is also polar, then the polar sample will most likely travel far.
Principle of “like dissolves like” in TLC: If water, methanol, ethanol, propanol, and acetone are the solvents used (MP), what will most likely happen to a polar sample? Will it travel far/close from the sample spot (starting point)? Why?
Petroleum ether, hexane, toluene, and dichloromethane (DCM) are nonpolar solvents. Since the SP (silica) is polar and the sample is polar, there is more attraction between the SP and the sample. The sample would not move with the nonpolar solvent, causing it to stay close from the starting point.
Principle of “like dissolves like” in TLC: If petroleum ether, hexane, toluene, and dichloromethane (DCM) are the solvents used (MP), what will most likely happen to a polar sample? Will it travel far/close from the sample spot (starting point)? Why?
Water, methanol, ethanol, propanol, and acetone are polar solvents. Since we are using a nonpolar stationary phase (C-18 has a nonpolar SP) and our sample is also nonpolar, then there is more attraction between the stationary phase and the sample, causing the sample to travel close from the sample spot.
Principle of “like dissolves like” in a C-18 (nonpolar SP): If water, methanol, ethanol, propanol, and acetone are the solvents used (MP), what will most likely happen to a nonpolar sample? Will it travel far/close from the sample spot (starting point)? Why?
The polar sample has a very poor affinity for the nonpolar mobile phase (petroleum ether). Because the sample is insoluble in the solvent, the mobile phase cannot transport it up the plate. The lack of solubility prevents any movement, causing the sample to remain at or very near the starting spot, regardless of its interaction with the stationary phase.
Principle of “like dissolves like” in C-18 (nonpolar SP): If petroleum ether, hexane, toluene, and dichloromethane (DCM) are the solvents used (MP), what will most likely happen to a polar sample? Will it travel far/close from the sample spot (starting point)? Why?
Since the SP is polar, the MP is nonpolar, and the sample is nonpolar, obviously there will be more attraction between the MP and the sample (following “like-dissolves-like”). Since it is attracted to the mobile phase, it will travel far from the starting point.
TLC uses silica or alumina as its stationary phase and these are polar. If you used a nonpolar solvent as your mobile phase (normal), and you have a nonpolar sample, what will most likely be the result?
P-anisaldehyde stain
The greatest advantage of this stain is that different colors are manifested on TLC on heating for different molecules. Therefore, molecules can be differentiated even if they have the same Rf values. The disadvantage is its strong but pleasant odor release during heating (toxic, in the hood!).
Cerium sulfate stain
Common TLC stains: General stain. Most compounds are stained brown or yellow.
Cerium molybdate stain
Common TLC stains: One of the most sensitive stains which detects most functional groups. The disadvantage is that everything stains blue.
Iodine/I2
Common TLC stains: Everything stains yellow. Solid I2 can be added to a developing chamber and the TLC plate developed by placing the plate in the chamber.
KMnO4
Common TLC stains: Detects molecules with an “oxidizable” functional group. Relatively insensitive, every- thing stains yellow, frequently even without heating.
Ninhydrin Solution
Common TLC stains: Especially sensitive to amino acids, as well as amines and anilines. Avoid contact with skin.
Phosphomolybdic acid (PMA)
Common TLC stains: Everything stains blue–green. A very sensitive stain, possibly used most often.
Vanillin Stain
Common TLC stains: Different colors are manifested on heating for different molecules.
UV Light
Specific Groups Detected by Stains: For aromatics + conjugated systems
Iodine
Specific Groups Detected by Stains: Visualizes ~half the time. Strongly reacts with aromatics.
p-Anisaldehyde and Vanillin
Specific Groups Detected by Stains: For many aldehydes, ketones, and alcohols, but it is very toxic (strong but pleasant odor)
Permanganate
Specific Groups Detected by Stains: For alkenes, alkynes, or oxidizable groups (aldehydes to COOH, alcohols to ketones/aldehydes)
Phosphomolybdic Acid (PMA)
Specific Groups Detected by Stains: For alcohols, phenols, alkenes, and many carbonyl compounds
Iron (iii) Chloride
Specific Groups Detected by Stains: For phenols
Bromocresol Green
Specific Groups Detected by Stains: For acidic compounds
Vanillin
Specific Groups Detected by Stains: For many aldehydes, ketones, and alcohols.
Column chromatography
Another form of solid-liquid adsorption chromatography and depends on the same fundamental principles as does thin-layer chromatography
Gas-liquid chromatography (GLC) or Gas chromatography
A technique that may be used to separate mixtures of volatile compounds whose boiling points may differ by as little as 0.5 °C.
components
pure
Gas chromatography can also be applied as an analytical tool to identify the _________ of a mixture or in preparative applications when quantities of the ____ components are desired (want to be found).
partitioning
mobile gaseous
stationary liquid
Gas-liquid chromatography operates on the principle of ________ the components of a mixture between a __________ phase and a ___________ phase.
helium or nitrogen
carrier
Gas-liquid chromatography: In practice, a sample is injected into a heated chamber where it is immediately vaporized and carried through a column by a flowing inert gas such as ______ and _______, which is called the _____ gas.
viscous, high-boiling liquid
stationary
Gas-liquid chromatography: The gaseous mixture is the mobile phase. The column is packed with a finely divided solid support that has been coated with a _____________, which serves as the _______ phase.
Fourier Transform Infrared Spectroscopy (FTIR)
A technique used to identify molecules by studying how they absorb infrared (IR) light