GENERAL SCIENCE 2ndQ
1/119
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
120 Terms
Chirality
non-superimposable
Molecular Asymmetry
In studying crystals of sodium ammonium tartrate, he discovered that although they had the same chemical composition, they did not necessarily have the same structure. He noted that the molecules occurred in two mirror-image arrangements that could not be superimposed.
is the foundation of stereochemistry. It had huge implications for how we understand such things as DNA.
Chemistry
The science that deals with the properties, composition, and structure of substances, the transformations they undergo, and the energy that is released or absorbed during these processes.
Louis Pasteur
Born on December 27, 1822, and died on September 28, 1895, is a French chemist and microbiologist who was one of the most important founders of medical microbiology. He has made great contributions in the field of science, technology, and medicine. In 1884, he became a professor of chemistry at the University of Strasbourg.
Achirality
superimposable
Fermentation
In mid-1850s, Pasteur undertook a series of studies on alcoholic fermentation at a local distillery. While doing so, he learned about many aspects of fermentation, including the compounds that cause milk to sour. In 1857, he presented evidence that all fermentation is caused by microorganisms. Specific organisms cause specific kinds of fermentation.
Pasteurization
Using his work with fermentation, Pasteur was able to devise a process known as pasteurization. It is a process that utilizes heat to kill microbes and preserve certain products. Pasteurization prevents fermenting and spoilage in beer, milk, and other goods.
Disproved Spontaneous Generation
Before Pasteur, many prominent scientists believed that life could arise spontaneously. For example, many people thought that maggots appeared from rotted flesh, and that dust created fleas. However, he disproved the idea by boiling broth in a special flask that deters contamination. When the broth was not exposed to air, it remained sterile and free of microorganisms. When the flask neck was broken and air was allowed to reach the broth, the fluid became cloudy with microbial contamination.
Germ Theory
He deduced that certain diseases result from invasion of the body by microorganisms. Before Pasteur, many people believed that all diseases came from inside the body rather than outside. His findings led to improvements in sterilizing and cleaning in medical practices, and antiseptic methods in surgery.
Infectious Diseases
Pasteur discovered the cause of a silkworm disease, threatening the French silk industry. He developed methods to preserve healthy silkworm eggs and prevent contamination, advancing epidemiology, and silk production
Vaccines
Using his germ theory, he developed vaccines for chicken cholera and anthrax, particularly the rabies vaccine, which helped in the development of preventive medicine in 1885.
Matter
anything that occupies space and has mass and volume.
Solid
Particles are tightly packed
Liquid
Particles are close together but loosely bonded.
Gas
Particles are far apart or scattered
Pure Substance
substances that are made up of only one kind of particle and have a fixed or constant structure.
Element
– a substance that consists of only one type or kind of atom.
Compound
– two or more elements are combined chemically in a fixed ratio.
Mixture
a combination of two or more substances that are not chemically bonded and retain their individual properties.
Homogeneous
– has a uniform appearance and composition throughout, with all components evenly distributed and indistinguishable.
Heterogeneous
- has visibly different components that are not uniformly distributed, allowing you to see the separate parts
Bleach
breaks down stains and kills bacteria through oxidation.
Active Ingredients: Sodium Hypochlorite (NaClO)
Calcium Hypochlorite [Ca(ClO₂)] in bleach
water purification
Chlorine Gas (Cl₂) in bleach
industrial bleaching
Chlorine Dioxide (ClO₂) in bleach
strong oxidizing agent
Detergent
removes dirt, grease, and stains from fabrics, dishes, and surfaces.
Active Ingredients: Surfactants and enzymes
Surfactants in detergent
lifts dirt and grease
Enzymes in detergent
break down protein-based stains
Builders in detergent
softens water and enhances cleaning power
Bleaching Agents in detergent
stain removal and disinfection
Fragrances and Dyes in detergent
adds scent and color
Baking Soda
a leavening agent, odor neutralizer, and mild abrasive for cleaning.
Active Ingredients: Sodium Bicarbonate (NaHCO₃)
Carbon Dioxide (CO₂) in Baking Soda
released in reactions, creating bubbles in baked goods
Air Freshener
neutralized odors and releases pleasant scents indoors.
Active Ingredients: Fragrances and Odor Neutralizers
Essential Oils in Air Freshener
provide scent
Propellants in Air Freshener
helps disperse fragrance
Odor Neutralizers in Air Freshener
absorb unwanted smells
Volatile Organic-Compounds (VOCs) in Air Freshener
found in some sprays, may affects air quality
Fabric Softeners
reduce static cling, softens fabrics, and adds fragrance to laundry
Active Ingredients: Cationic Surfactants
Fragrances in Fabric Softeners
provides a fresh scent
Silicones and Conditioning Agents in Fabric Softeners
smooths fabric texture
Preservatives and Stabilizers in Fabric Softeners
maintains product effectiveness over time
Shampoo
cleanses, nourishes, and protects hair and scalp.
Active Ingredients: Surfactants (Sodium Lauryl Sulfate, Ammonium Laurate Sulfate)
Water in shampoo
main base ingredient
Conditioning Agents in shampoo
softens and detangles (Silicones, Fatty Alcohols)
Preservatives in shampoo
prevent bacterial growth (Parabens, Benzyl Alcohol)
Fragrances and Additives in shampoo
adds scent and UV protection
Soap
removes dirt, oil, and bacteria; hydrates and protects skin.
Active Ingredients: Sodium Hydroxide (NaOH), Potassium Hydroxide (KOH)
Fatty Acids and Oils in soap
base ingredients (Coconut Oil, Olive Oil)
Glycerin in soap
retains moisture
Fragrances and Essential Oils in soap
add scent and skin benefits
Antibacterial Agents in soap
helps fight germs (Triclosan)
Toothpaste
cleans, protects, and maintains oral health
Active Ingredients: Fluoride (Sodium Fluoride, Stannous Fluoride)
Abrasives in toothpaste
removes plaque (Calcium Carbonate, Hydrated Silica)
Humectants in toothpaste
prevents drying out (Glycerin, Sorbitol)
Detergents in toothpaste
create foam (Sodium Lauryl Sulfate)
Antibacterial Agents in toothpaste
prevents gum disease and bad breath (Triclosan, Stannous Fluoride)
Flavoring Agents in toothpaste
enhance taste (Mint, Sweeteners)
Chemical Reaction
a process in which one or more substances are converted to one or more different substances.
signs/signages
serve as a warning for possible hazards that might occur
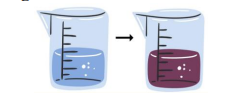
Change of color
Evidence of Chemical Reaction (rusting)
often caused by the formation of a new substance with different electronic properties, leading to the absorption and reflection of different wavelengths of light
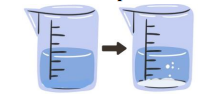
Formation of Gas
Evidence of Chemical Reaction(Acid-carbonate reaction)
is a chemical reaction that produces a gas, which is often observed as bubbles
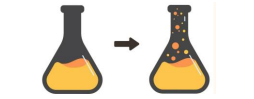
Formation of Precipitate
Evidence of Chemical Reaction
two aqueous solutions are mixed, and a solid, insoluble compound called a precipitate forms.
Production of Light and Change in Temperature
Evidence of Chemical Reaction (striking a match)
is an exothermic reaction, specifically a combustion reaction. In this type of reaction, energy is released into the surroundings, causing a temperature increase and, in many cases, a visible flame.
Chemical Equation
a symbolic representation of a chemical reaction.
Law of Conservation of Mass
The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another.
Reactants
nasa left side ng chemical equation
Product
nasa right side ng chemical equation
Synthesis/Combination
A reaction in which two or more substances combine to form a single new substance. (A + B → AB)
Decomposition
A reaction in which a compound breaks down into two or more simpler substances. Most decomposition reactions in the form of heat, light, or electricity (ABCD → A + B + C + D)
Acid – Carbonate general qs
is a neutralization reaction where an acid and a carbonate combine to form a salt, water, and carbon dioxide gas, which causes the mixture to fizz. This reaction is considered a neutralization because the carbonate, a basic substance, neutralizes the acidic properties of the acid, resulting in products closer to a neutral pH.(Acid + Carbonate [Base] → Salt + Water + Carbon Dioxide)
Acid – Metal
is a chemical reaction where a reactive metal and an acid combine to form a new chemical compound called a salt and hydrogen gas. The metal essentially “replaces” or “displaces” the hydrogen in the acid, a process known as a single displacement reaction.(Acid + Metal → Salt + Hydrogen Gas)
Combustion
A reaction in which a substance (usually a fuel) reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions must involve O2 as one reactant. The combustion of hydrogen gas produces water vapor. (Fuel + O2 → CO2 + H2O + Energy)
synthesis/combination example
4Fe + 3O₂ → 2Fe2O3
rust formation
Decomposition example
2NaHCO3 → Na2CO3 + H2O + CO2
baking
Acid – Carbonate
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
cleaning
Acid – Metal ex
Zn + 2HCl → ZnCl2 + H2
metal cleaning
Combustion example
CH4 + 2O2 → CO2 + 2H2O + Heat
combustion of methane
Photosynthesis
Formula: Carbon Dioxide + Water + Light Energy → Glucose + Oxygen
Occurs when plants use light energy to convert CO2 and water into glucose and oxygen.
Chloroplasts
Refers to an organelle in plant and algae cells that performs photosynthesis, converting sunlight, water, and CO2 into energy-rich sugars and oxygen
Photo
Light
Synthesis
Combining Together
Chlorophyll
a green-colored pigment that absorbs light energy
stomata
tiny pores located in the epidermis of leaves where CO2 enters
cellulose and starch
more complex carbohydrates that form when sugar molecules combine with ea
Cellulose
is considered the structural material that is used in plant cell walls.
Cellular Respiration
Formula: Glucose + Oxygen → CO2 + Water + ATP
A Metabolic process that occurs in all organisms. From singlecelled organisms to dominant multicellular organisms. It is a biochemical process that occurs within the cells of an organism.
ATP (Adenosine triphosphate)
produced in cellular respiration by the breakdown of glucose, which is further used by cells to perform various functions.
Aerobic
A type of cellular respiration taking place in the presence of oxygen to produce energy. A continuous process that takes place within the cells of animals and plants.
Anaerobic
A type of cellular respiration taking place in the absence of oxygen to produce energy. (Bacteria, archaea, fungi, and yeast.)
Metabolism
It is the process by which the body turns food and beverages into energy that makes our body alive and functioning.
homeostasis
regulates metabolism to keep it balanced within a narrow range that allows enzymes to work and cells to survive
Catabolism
The process of breaking down big, complex molecules into smaller ones, so that they’ll be easier to absorb. In other words, POTENTIAL energy is changed to KINETIC energy. This process releases energy; hence, ATP is on the product side. Thus, it is important for living entities to perform different activities.
ex. of catabolism
o Protein → Amino Acids
o Glycogen → glucose o Triglycerides → fatty acids
o C6H12O6 + 6O2 → 6CO2 + 6H2O + ATP (Cellular Respiration)
o C6H12O6 → 2C3H6O3 (lactic acid) + 2ATP [Lactic acid fermentation]
Anabolism
This process builds molecules required for the body’s functionality (e.g., maintenance, growth, and storage). This also means that KINETIC energy is converted into POTENTIAL energy. Hence, it requires energy. (In other words, energy is on the reactant’s side).
adrenaline, glucagon, cortisol, and cytokine.
hormones involved in catabolism
estrogen, testosterone, growth hormones, and insulin.
hormones involved in anabolism
ex. of anabolism
o Amino acids → Polypeptides
o Glucose → Glycogen o Fatty acids → Triglycerides
o 6CO2 + 6H2O + light energy → C6H12O6 + 6CO2 (Photosynthesis)
o Amino Acids + ATP + GTP → Polypeptide Chain (Protein Synthesis)
Carbohydrate
It serves as a primary energy source.
It has two different paths: Glucose is broken down in catabolism to release energy in the form of ATP. Or, it is stored as glycogen (Anabolism) when not immediately used.
Protein
It is a structural/building material (Anabolism). It serves as a backup energy source when carbs/fats are insufficient. It also has two different pathways: Amino acids build new proteins, such as muscle fibers, enzymes, hormones, etc. (Anabolism), or amino acids can be deaminated, converted into pyruvate or intermediates for the Krebs’ cycle