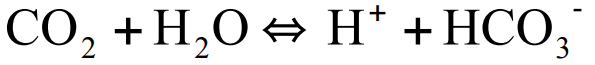Haemoglobin & Myoglobin
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Haeme definition
A prosthetic group at the site of O₂ binding
Apoprotein
Haeme without a prosthetic group
Holoprotein
Haeme with a prosthetic group
Haeme structure (7)
Contains protoporphyrin IX with an Fe atom in its center
The Fe atom is in the ferrous (Fe²⁺) oxidation state in functional myoglobin
The Fe atom can form 5 or 6 ligand bonds
depending on O₂ binding
Four bonds are to the pyrrole nitrogen atoms of the porphyrin, lying in the plane of the ring
The 5th and 6th bonds are directed perpendicular to the porphyrin ring
The 5th coordinate bond is to a nitrogen of histidine imidazole (proximal histidine)
The 6th coordinate bond is to O₂ or another histidine (distal histidine)
Proximal Histidine
5th coordinate bond is to a nitrogen of histidine imidazole
Distal Histidine
6th coordinate bond is to O₂ or another histidine
Porphyrin Hydrophobic Pocket (5)
Haeme is positioned within a hydrophobic pocket of each globin subunit
Non-covalent interactions with approximately 18 residues
mainly from apolar side chains + apolar regions of the porphyrin
stabilize the haeme
80 interactions
Driving Force for Non-covalent Interactions
The expulsion of H2O of solvation on association of the hydrophobic haeme with the apolar a.a. side chains in the haeme pocket
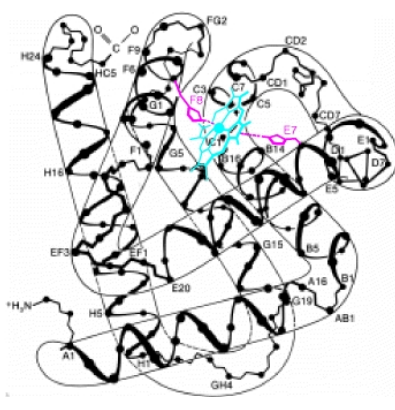
Myoglobin characteristics (6)
153 amino acids in the polypeptide chain
83 invariant residues
with 15 identical to mammalian haemoglobin
A single polypeptide chain with one O2 - binding site
Binds and releases O2 with changes in the O2 concentration in skeletal muscle cells' sarcoplasm
Acts as a monomer, preventing other myoglobin molecules from associating
Function of Myoglobin (2)
Binds and releases O₂ based on changes in the O₂ concentration in skeletal muscle cells
Essential for oxygen storage in muscle tissue
Myoglobin 2° Structure (2)
Approximately 70% of residues form α-helical structures, generating:
Seven helical segments in its structure
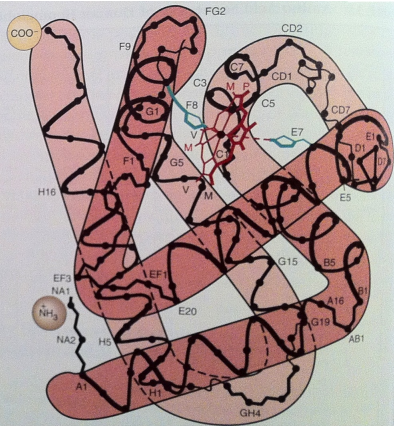
Haemoglobin Characteristic (3)
It’s surface residues are designed to form H bonds and nonpolar contacts with other subunits
Supporting its quaternary (4°) structure
Tetrameric - interact more extensively with each other
Haemoglobin 2° Structure (3)
Approximately 70% of residues form α-helical structures, generating:
Seven helical segments in the α chain
Eight helical segments in the β chain
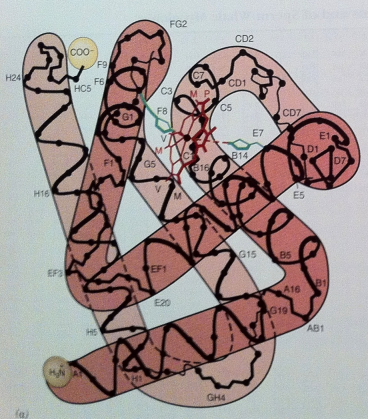
Helical Regions - M & H (3)
The interhelical regions are labeled AB, BC, CD, …, GH
The nonhelical region
at the NH₂-terminal end is the NA region
at the COOH-terminal end is the HC region
General Properties for M & H (4)
Changes in amino acid sequences are conservative
preserving the physical properties of residues
The 2° and tertiary (3°) structures of hemoglobin subunits and myoglobin are nearly identical
Physiological differences arise from small specific changes in structure
The similarity in tertiary structure shows that the same 3° structure can result from diverse amino acid sequences
Oxygen Binding and Equilibrium in Myoglobin (3)
Follows a simple equilibrium constant (Keq)
which depends on pH, ionic strength, and temperature
[Keq] = mol/L
![<ul><li><p>Follows a simple equilibrium constant (K<sub>eq</sub>)</p><ul><li><p>which depends on <strong>pH, ionic strength, and temperature</strong></p></li></ul></li><li><p>[K<sub>eq</sub>] = mol/L</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/1e9fb1d4-652a-477e-adee-7707e043e1aa.png)
P50 definition
The equilibrium constant representing the O₂ partial pressure at which 50% of the oxygen-binding sites are occupied
P50 equation (3 + photo!)
The pressure of O₂ is measured in torr
as its partial pressure
1 torr = pressure of 1 mmHg at 0°
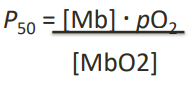
pO2 definition
The concentration of O₂ expressed in torr, used to describe the O₂-binding dynamics of proteins like myoglobin
O₂ Saturation Curve (3+photo!!)
Used to characterize the properties of an O₂-binding protein
It plots the fraction of O₂-binding sites occupied (Y) on the ordinate (y-axis)
Versus the partial pressure of O₂ (pO₂) on the abscissa (x-axis)

Y (Fractional Saturation) of Myoglobin (2 + photo!!)
[MbO2] is the concentration of oxygen-bound myoglobin
[Mb] is the concentration of unbound myoglobin
![<ul><li><p>[MbO2] is the concentration of oxygen-bound myoglobin</p></li><li><p>[Mb] is the concentration of unbound myoglobin</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/92541e61-49ad-4d7c-801b-2ad1f111b535.png)
Simplified Equation for Fractional Saturation (Y) (5 + photo!!)
By dividing through by [Mb]
Where:
Y: fractional saturation
P50: equilibrium constant
pO2: oxygen concentration in terms of partial pressure
![<ul><li><p>By dividing through by [Mb]</p></li><li><p>Where:</p><ul><li><p>Y: fractional saturation</p></li><li><p>P<sub>50</sub>: equilibrium constant</p></li><li><p>pO<sub>2</sub>: oxygen concentration in terms of partial pressure</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f77ca209-518f-4380-87bc-122df6807cf0.png)
P50 = pO2 when? (3)
when Y=0.5
indicating 50% of available binding sites are occupied
This defines the equilibrium constant's designation as P50 with the subscript "50" referring to 50% saturation.
Saturation Curve Characteristics (3)
A plot of Y versus pO2 generates the oxygen saturation curve for myoglobin
The curve takes the shape of a rectangular hyperbola
Reflects the direct dependence of fractional saturation on oxygen concentration and P50
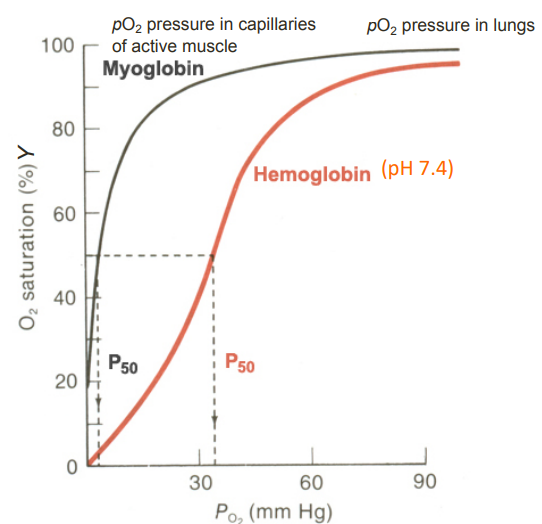
O2-binding curves for Myoglobin and Haemoglobin (photo!)
Rearranged O₂-binding Equation (photo!)
A simple algebraic manipulation of the equation used to construct the O₂-binding curve
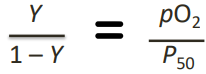
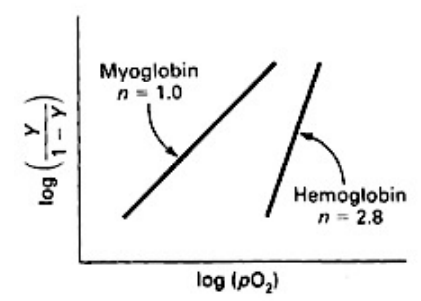
Hill Equation - Logarithmic Form
Used to assess the cooperativity of oxygen binding to a molecule
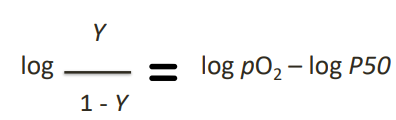
Hill Coefficient - Hill Plot (4)
photo* produces a straight line
Hill coefficient (nH):
the slope of that straight line
reflects the cooperativity of oxygen binding
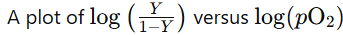
Cooperativity in Myoglobin (3)
Myoglobin has a single O₂-binding site per molecule
It does not exhibit cooperativity
Hill coefficient is 1
O₂ Saturation Curves - Myoglobin (2)
Hyperbolic
Reflecting the simple binding of O₂ without cooperativity
Cooperativity in Haemoglobin (3)
Hb has 4 monomeric subunits, each with an O₂-binding site (4)
The binding of O₂ to one subunit enhances the binding of O₂ to the other subunits
Hill coefficient >1 - reflecting this cooperative behavior
O₂ Saturation Curves - Haemoglobin (4)
Sigmoidal
indicating cooperative binding of O₂
Steep in the middle
demonstrating the enhanced affinity for O₂ as more O₂ molecules bind
T-State (5)
= Quaternary structure of deoxyhaemoglobin
Lower oxygen affinity
Tense conformation
Stabilized by salt bridges
Hydrogen bonds
Iron Position
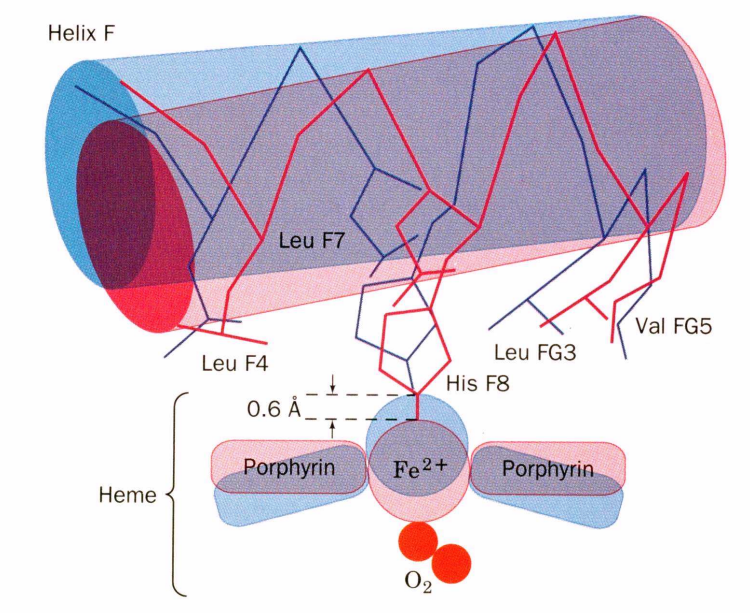
In the deoxy state, the Fe is 0.6 Å out of the heme plane (angstrom)
R-State (4)
= Quaternary structure of oxyhaemoglobin
Higher oxygen affinity
Relaxed conformation
Induced by oxygen binding
Oxygenation rotation (2)
Rotates the α1β1 dimer relative to the α2β2 dimer by 15°
Oxygen binding to one subunit facilitates binding to others
Positive Cooperativity of O2 binding definition
Arises from the effect of one heme's ligand-binding state on the affinity of another
Oxygen Binding
Oxygen pulls the iron back into the heme plane
F Helix Movement
The proximal histidine (His F8) attached to the iron pulls the entire F helix, acting like a lever on a fulcrum
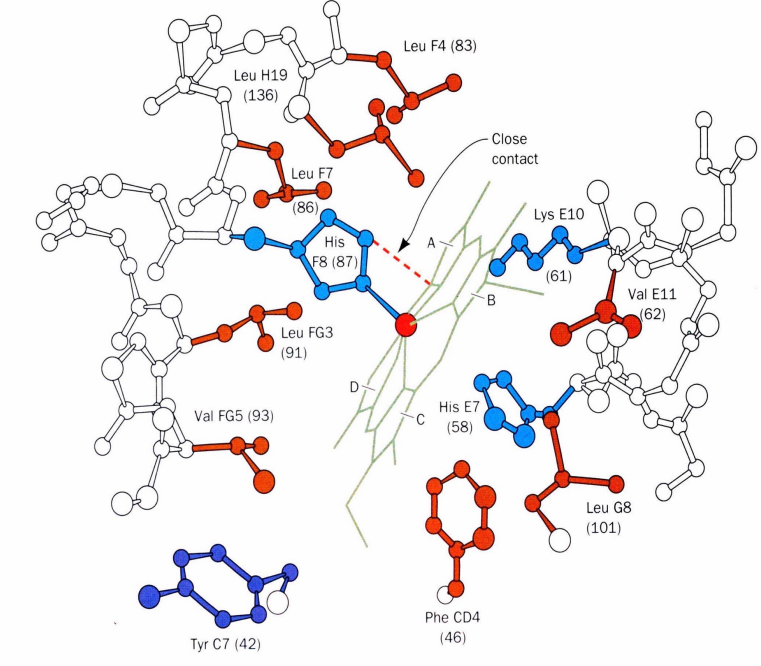
Positive Cooperativity of O2 binding (photo!)
Oxygen Binding and Affinity Change (5)
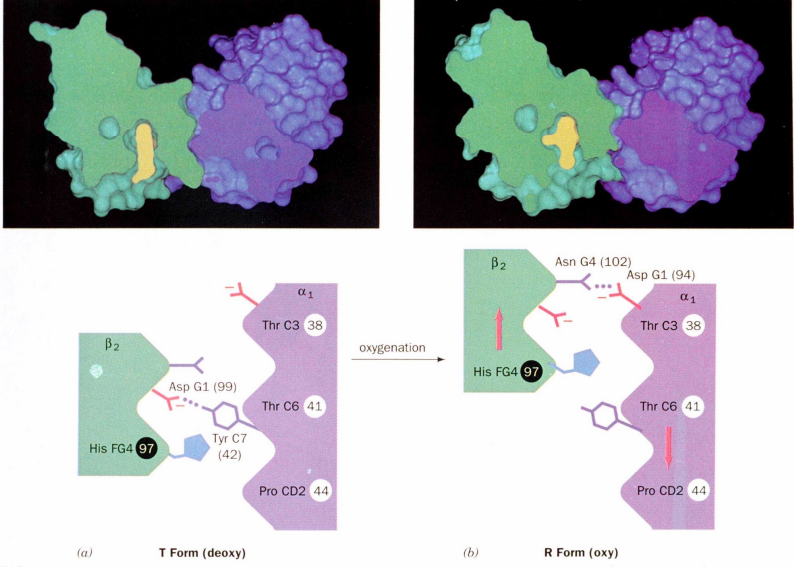
Binding of oxygen to one heme group in hemoglobin is initially more difficult
However, oxygen binding causes a shift in the α1-β2 contacts
This shift moves the distal histidine (His E7) and Val E11 out of the oxygen's path
facilitating oxygen binding to the Fe2+ in the other subunit
As a result, the affinity of the heme for oxygen increases
α1-β2 Contacts and Structural States (3)
The α1-β2 contacts have two stable positions
These contacts, held together by different but equivalent hydrogen bonds
act as a binary switch between the T (tense) and R (relaxed) states of hemoglobin
Transition Between States (8)
The energy from the formation of the Fe²⁺-O₂ bond drives the T → R transition
Hemoglobin's O₂-binding cooperativity arises from this transition
The Fe²⁺ of any subunit cannot move into its heme plane without the reorientation of its proximal histidine (His)
prevents this residue from bumping into the porphyrin ring
The proximal His is tightly packed by surrounding groups
making reorientation impossible
unless accompanied by the translation of the F helix across the heme plane
The F helix translation is only possible in concert with the quaternary shift that steps the α1C-β2FG contact one turn along the α1C helix
Methemoglobin (3)
Produced when Fe(II) to Fe(III) oxidized
Brown
Sixth position is coordinated with water instead of oxygen
Brown Color of Methemoglobin (2)
Visible in dried blood and old meat
Contributes to less desirable appearance of aged meat
Methemoglobin vs Ascorbic Acid (3)
Butchers' use Ascorbic Acid
Reduces methemoglobin back to hemoglobin
Helps maintain fresher appearance of meat
Enzyme Involvement - Methemoglobin (2)
Methemoglobin reductase converts methemoglobin to regular hemoglobin
Restores hemoglobin's ability to bind and carry oxygen
Bohr Effect (2+2)
Higher pH (lower [H+])
Promotes tighter binding of oxygen to hemoglobin
Lower pH (higher [H+])
Permits easier release of oxygen from hemoglobin
![<ul><li><p>Higher pH (lower [H<sup>+</sup>])</p><ul><li><p>Promotes tighter binding of oxygen to hemoglobin</p></li></ul></li><li><p>Lower pH (higher [H<sup>+</sup>])</p><ul><li><p>Permits easier release of oxygen from hemoglobin</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/3891562b-0641-4b9c-9aae-8c9532341f56.png)
P50 and pH Relationship (2)
As pH increases, P50 value decreases, indicating increased oxygen binding
As pH decreases, P50 value increases, indicating decreased oxygen binding
Effect of pH on Oxygen Release
At 20 torr, 10% more oxygen is released when pH drops from 7.4 to 7.2
Carbonic Anhydrase (2)
Catalyzes the conversion of CO2 to bicarbonate (HCO3-) in red blood cells
As oxygen is consumed, CO2 is released