(g) acids, bases and salt preparations
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
solubility rules (2.34)
common sodium, potassium and ammonium compounds are soluble
all nitrates are soluble
common chlorides are soluble, except those of silver and lead(II)
common sulfate are soluble, except for those of barium, calcium and lead (II)
common carbonates are insoluble, except for those of sodium, potassium and ammonium
common hydroxides are insoluble except for those of sodium, potassium and calcium (calcium hydroxide is slightly soluble)
acids and bases in terms of proton transfer (2.35 / 2.36)
an acid is a proton (H+) donor and a base is a proton (H+) acceptor
a proton is the same as a hydrogen ion. a good way to think about that is to realise that a hydrogen atom is just one proton and zero neutrons surrounding by only one electron. if that atom becomes an ion by the removal of the electron, then only one proton is left.
when sulfuric acid reacts with copper (II) oxide (CuO):
Cu2+O2-(s) + H2SO4 (aq) → Cu2+(aq) + SO42-(aq) + H2O(l)
H2SO4 is an acid and it donates protons (H+) to CuO, the base
acid reactions (2.37)
alkali + acid → water + salt (AAWS)
base + acid → water + salt (BAWS)
carbonate + acid → water + salt + carbon dioxide (CAWSCD)
metal + acid → salt + hydrogen (MASH)
AAWS / BAWS
alkalis are soluble bases. when they react with acids, a salt and water is formed. the salt formed is often as a colourless solution. alkalis are a source of hydroxide ions (OH-) when in solution
e.g.
sodium hydroxide + hydrochloric acid → sodium chloride + water
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
potassium hydroxide + sulfuric acid → potassium sulfate + water
2KOH(aq) + H2SO4 (aq) → K2SO4 (aq) + 2H2O (l)
CAWSCD
a carbonate is a compound made up of metal ions and carbonate ions. examples of metal carbonates are sodium carbonate, copper carbonate and magnesium carbonate.
when carbonates react with acids, bubbling is observed which is the carbon dioxide being produced. if the acid is in excess the carbonate will disappear.
e.g.
calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide
CaCO3 (s) + 2HCl (aq) → CaCl2 (aq) + H2O (l) + CO2 (g)
potassium carbonate + hydrochloric acid → potassium chloride + water + carbon dioxide
K2CO3 (aq) + 2HCl (aq) → 2KCl (aq) + H2O (l) + CO2 (g)
MASH
metals will react with an acid if the metal is above hydrogen in the reactivity series.
when metals react with acids, bubbling is observed which is the hydrogen being produced. if the acid is in excess the metal will disappear
e.g.
magnesium + sulfuric acid → magnesium sulfate + hydrogen
Mg (s) + H2SO4 (aq) → MgSO4 (aq) + H2 (g)
aluminium + hydrochloric acid → aluminium chloride + hydrogen
2Al(s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2 (g)
what is a base (2.38)
a base is a substance that neutralises an acid by combining with the hydrogen ions in them to produce water. this usually means a metal oxide, a metal hydroxide or ammonia.
an alkali is a base that is soluble in water.
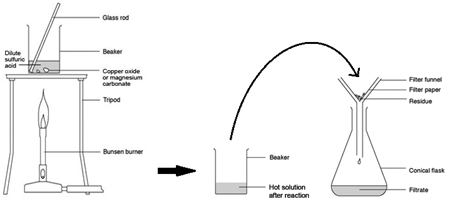
describe an excess insoluble base experiment (2.39 / 2.42)
method: excess insoluble base (used when the salt is soluble but not a sodium or potassium salt)
copper oxide + sulfuric acid → copper sulfate + water
CuO(s) + H2SO4 (aq) → CuSO4 (aq) + H2O(l)
1) heat the acid (H2SO4) in a beaker to speed up the rate of reaction
2) add the base (CuO), stirring with a glass rod until it is in excess (aka no more copper oxide will dissolve, meaning there is solid collecting at the bottom of the beaker) to neutralise all the acid
3) filter the mixture using filter paper and a funnel to remove the excess copper oxide
4) gently heat the filtered solution in an evaporating basin to evaporate some of the water until the solution is saturated
5) leave the solution to crystallise
6) rinse with distilled water
(heat, dissolve till excess, filter, heat, evaporate, rinse)
describe a titration (2.40C)
method: titration (used when the salt is soluble and is a sodium or potassium salt)
hydrochloric acid + sodium hydroxide → sodium chloride + water
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
1) pipette 25cm3 of the alkali (NaOH) into a conical flask and add a few drops of phenolphthalein
2) fill a burette with acid and take note of the reading
3) add just enough acid for the solution to go colourless
4) take note of the difference in the volume of the acid
5) do the experiment again without any phenolphthalein to avoid the contamination of the crystals
6) evaporate water to make a saturated solution
7) crystallise the solution and leave to dry
(25cm3 of alkali + indicator, acid in burette, mix till neutralised, note difference, reapeat - indicator, evaporate, crystallise, dry)
describe a precipitation (2.41C)
method: precipitation (used when the salt is insoluble)
silver nitrate + potassium chloride → silver chloride + potassium nitrate
AgNO3 (aq) + KCl (aq) → AgCl (s) + KNO3 (aq)
1) mix your two salt solutions together to form a precipitate of an insoluble salt (one solution is acidic (H+) and one is alkaline (OH-))
2) filter, keep the precipitate
3) rinse the precipitate with distilled water
4) dry
(mix solutions, filter, rinse, dry)
prepare a sample of pure, dry lead (II) sulfate (2.43C)
method: precipitation
lead (II) nitrate + sodium sulfate → lead (II) sulfate + sodium nitrate
Pb(NO3)2 (aq) + Na2SO4 (aq) → PbSO4 (s) + 2NaNO3 (aq)
1) mix your two salt solutions (lead (II) nitrate and sodium sulfate) together to form a precipitate of an insoluble salt
2) filter, keep the precipitate (lead (II) sulfate)
3) rinse the precipitate with distilled water
4) dry
(mix solutions, filter, rinse, dry)