PTE 731: exam 2
1/193
Earn XP
Description and Tags
module 2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
194 Terms
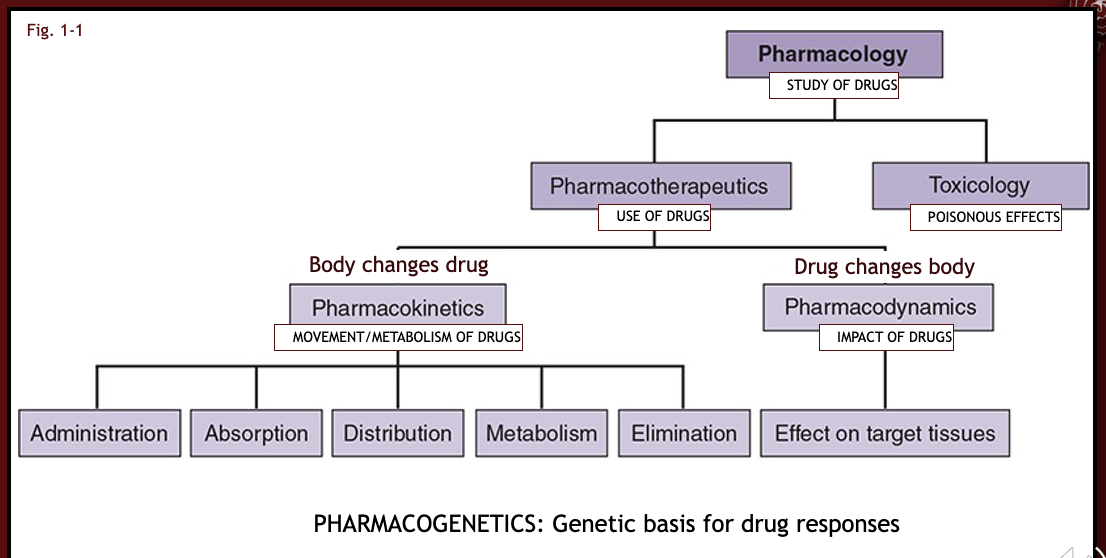
what is pharmacology?
study of drugs (any medicine or other substance which has a physiological effect when ingested or otherwise introduced into the body)
what is pharmacy?
preparation and dispensing of medications
what is pharmacotherapeutics?
area of pharmacology that refers to the use of specific drugs to prevent, treat, or diagnose a disease
what is pharmacokinetics?
the study of how the body absorbs, distributes, metabolizes, and eliminates the drug (key is movement)
what is pharmacodynamics?
the analysis of what the drug does to the body, including the mechanism by which the drug exerts its effects (key is impact)
what is pharmacogenetics?
the genetic basis of drug responses (key is inherited uniqueness)
what is toxicology?
study of harmful effects of drugs (key is poisonous effects)
what is a drug?
any substance that, when taken into a living organism, may modify one or more of its functions
what are the two categories of drugs?
legal (prescription and over the counter)
illegal (street and diverted)
T or F: drugs are unique in that they can heal and what they can’t heal, they can control, BUT they can always cause harm.
T; harm = allergic reactions, side effects, and/or other drug-drug interactions
______ _______ deals primarily with the beneficial effects of specific drugs on humans and the manner in which these drugs exert their therapeutic effects.
clinical pharmacology
T or F: pharmacology affects our patients but does not affect our therapy treatments.
F; affects both patient and therapy treatments
flow chart of definitions
what are the three classifications of a drug’s name?
chemical name
generic name
brand name
describe chemical name.
the biochemical description of the compound
describe generic name.
the official nonproprietary name for the chemical compound
there is only one generic name; NEVER changes
describe brand name.
the name the pharmaceutical company puts on the drug they have produced; each manufacturer has a different name
what classification of drug ends with the suffix “pril”?
ACE inhibitors (they block the activity of an enzyme called angiotensin-converting enzyme (ACE) preventing the conversion of angiotension-I to angiotension-II in the lungs)
why might a patient be prescribed an ACE inhibitor?
if the patient has a diagnosis of HTN, CHF, post-MI, DM, and/or renal failure
some side effects that commonly present with the use of ACE Inhibitors are cough, rash, hyperkalemia, hypotension, and angiodema. how might these side effects impact a patient’s treatment?
they can lead to exercise intolerance, fatigue, or any other issues associated with the side effects.
T or F: generic drugs are not the same as brand-name drugs, but they can be substituted for the brand-name drug if they are bioequivalent.
T
for a generic drug and a brand-name drug to have bioequivalence, they must have the same:
type and amount of the active ingredient (same compound in same dose)
administration route
pharmacokinetic profile
therapeutic outcomes
in the US, it is the _____ responsibility for monitoring the use of existing drugs and for developing and approving new drugs.
FDA’s (food and drug administration)
where does the preclinical stage of drug development occur?
in the laboratory (cellular or animal)
what are the four phases of clinical testing?
safety (healthy)
effectiveness (patient)
generalizability
ongoing monitoring
describe the first phase of clinical testing: safety
small number of healthy volunteers
duration: less than or equal to 1 year
aim: to determine drug’s effects, safety, dosage, pharmacokinetics
describe the second phase of clinical testing: effectiveness
limited number of patients with target disorder
duration: 2 years
aim: assess effect on disease/disorder
describe the third phase of clinical testing: generalizability/diversity
larger number of patients targeted
duration: 3 years
aim: assess safety and effectiveness in a larger patient population
describe the fourth phase of clinical testing: monitoring
general patient population
duration: indefinite
aim: monitor for any problems that may occur
in what instance would a drug be fast tracked and expedited through the clinical testing phases?
in an event of a serious and life-threatening condition
benefits over existing treatments or no drug available
fast track review, priority review, and accelerated approval
ex: COVID vac, cancer, and AIDS
what are orphan drugs?
drugs developed to treat rare disease affecting fewer than 200,000 people in the US
orphan drugs often face challenges in development due to limited patient populations and high costs, but special provisions and funding exist to support their development
T or F: drugs are categorized as prescription or over-the-counter based on their safety and required supervision.
T
describe over-the-counter (OTC) drugs.
used for minor/self-limiting conditions: cold/flu, headache, allergies
used to make the patient more comfortable until condition resolves → focuses on the dis-ease
generally safe for use without direct medical supervision → chances of toxic effects are usually small when taken in the recommended amounts
typically less expensive
T or F: OTC medications are used for minor/self-limiting conditions so they are safe to take in congruence with prescription medications.
F; inappropriate OTC use can cause serious interactions with prescribed meds
describe prescription drugs.
address more serious conditions
potentially serious adverse effects
what’s the difference between “on label” and “off label” prescribing patterns?
on label: using a drug in the manner of and for conditions approved and dictated by the NDA
off label: using a drug to treat conditions other than those for what it was created for; dictated by clinical observations and post-marketing studies
what are controlled substances?
refer to substances that are tightly controlled by the government bc they may be abused or cause addiction/dependence
what three factors are critical in determining the appropriate drug and dosage for a patient?
understanding dose-response relationships
potency
max efficacy
what are the four basic concepts of drug therapy?
drug must reach a target
drug must be able to change the function of their target in a way to help restore normal physiological function or prevent disease
dose must be large enough to produce a concentration sufficient to reach target and produce response
dose must not be so excessive that it produces toxic effects
dose vs. response curve graph
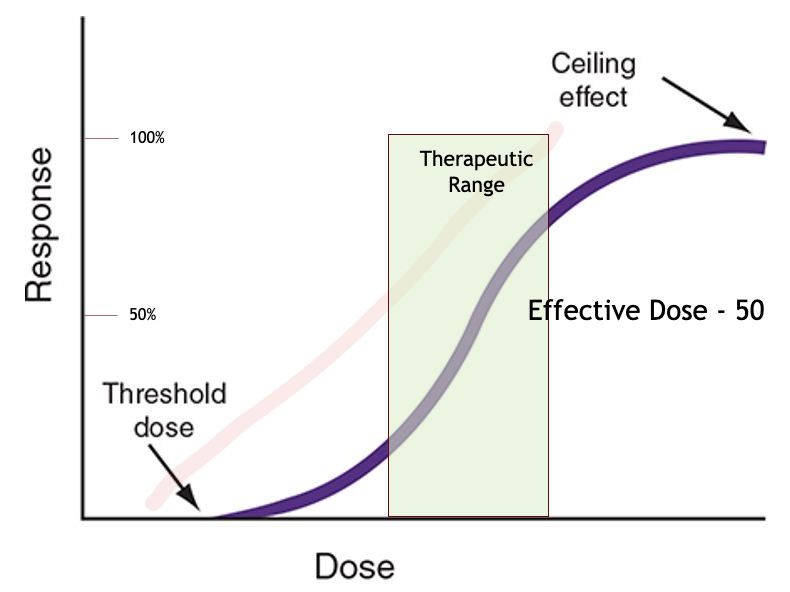
what is threshold dose?
the dosage needed to get any measurable response
this point marks the minimum effective dose of the drug
what is the ceiling effect?
the point where the drug dosage reaches its maximal efficacy
what effect does a drug have if it’s dosage is increased past its ceiling effect?
nothing good
increasing the dosage further does not increase the effect, but instead can lead to toxicity and lethality
what is potency?
the amount of drug needed to produce a measured effect
T or F: a more potent drug produces the same effect as a higher dose of a less potent drug.
T
more potent ____ ____ more effective.
doesn’t equal
what is the quantal dose-response curves?
these curves measure the percentage of a population that responds to a specific dose of drug, rather than the magnitude of response in an individual
two dose-response curves picture
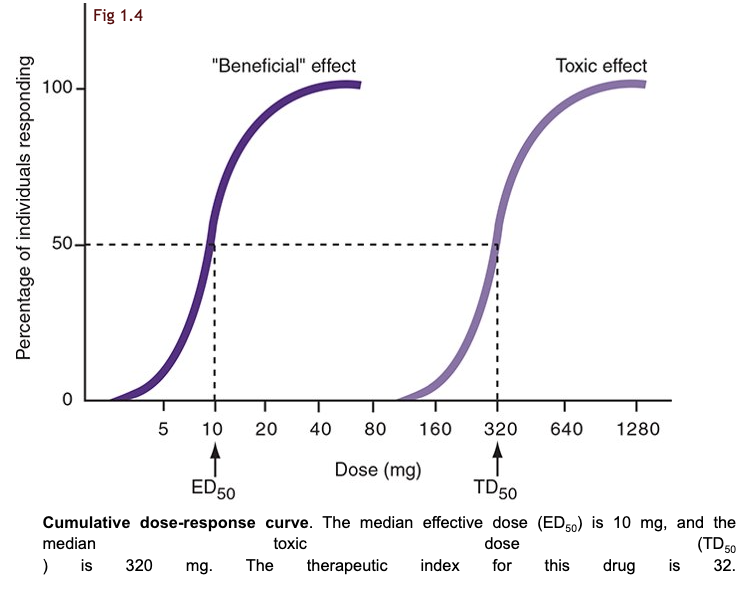
what is the median effective dose (ED50)?
the dose at which 50% of the population experiences the drug’s intended effect (50% will respond to drug)
useful in determining the effective of a drug across a varied population
what is the median toxic dose (TD50)?
the dose at which 50% of the population experiences a specific toxic effect (50% will show toxicity)
assess the potential risks associated with higher doses of a drug
what is the lethal dose (LD50)?
represents the dose that causes death in 50% of the animals tested
50% of patients will die
define the therapeutic index (TI).
TI is the ratio of TD50 to ED50 and indicates a drug’s safety
higher TI = _____ drug
safer
lower TI = ____ drug
risker/toxic
why does a higher TI indicate a safer drug?
it suggests a larger margin between effective and toxic doses
if a patient must take a drug with a low TI, how is she monitored?
via blood levels, clinical assessments, and physiological testing
what are the four elements of pharmacokinetics?
absorption/administration: getting in
distribution: dispersion to body fluids/tissues
metabolism: transformation to another form
elimination/excretion: removal from the body
what are the two ways a drug can be administered/absorbed?
enteral: oral, sublingual, and rectal
parenteral: inhalation, injection, transdermal, and topical
describe the advantages and disadvantages for oral medications.
advantages:
ease, safety, convenience, and good acceptance
disadvantages:
erratic absorption, first-pass effect
which enteral administration is most common and convenient?
oral
describe the advantages and disadvantages for sublingual medications.
advantages:
rapid onset, bypasses first-pass effect, don’t have to swallow, and good acceptance
disadvantages:
limited choices
why would a pharmacist prescribe a sublingual (under the tongue) drug to a patient?
it is useful if the drug would be degraded in the stomach (via first-pass effect) or if it requires quick onset
describe the advantages and disadvantages for rectal medications.
advantages:
alternative, added local effect, don’t have to swallow
disadvantages:
poor acceptance, erratic absorption, and local irritation
what types of patient statuses would benefit from a rectal medication?
unconscious or vomiting patients
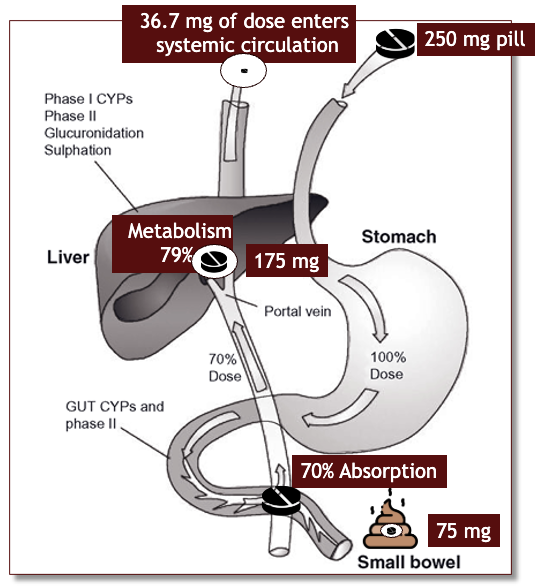
what is the first-pass effect?
when the drug is metabolized in the liver before reaching systemic circulation
what is bioavailability?
the extent to which the drug reaches the systemic circulation; expressed in it’s percentage of the amount that reaches the blood stream
bioavailability picture
describe the parenteral medication administered via inhalation.
inhalation delivers drugs directly to the lungs, offering rapid onset due to the large surface area of the alveoli
what are the characteristics that make up inhalation.
alveolar surface
variable delivery
rapid onset
may induce cough, irritation, infection, and/or dysphonia
describe the parenteral medication administered via injection.
direct delivery to circulation/tissue with reliable dosing via intravenous (IV), intramuscular (IM), subcutaneous (SC), intra-arterial, etc.
what are the risks associated with injections?
infection
bleeding
tissue damage
pain
describe the parenteral medication administered via transdermal.
provide a controlled release of drugs though the skin and interactions with subcutaneous enzymes, allowing for prolonged effects without the need for frequent dosing.
where are prime locations for the uptake of a transdermal medication?
upper arm, upper chest, and upper back
describe the parenteral medication administered via topical/cutaneous.
targets the skin or mucous membranes, offering localized effects with minimal systemic absorption
what is the function of membranes?
keep the inside in and the outside out
what is the structure of membranes that allow for composition and transport?
phospholipid bilayer and membrane proteins
T or F: lipid soluble compounds pass freely through the membranes by dissolving in the lipid bilayer.
T
the lipid layer is essentially ________ to water and other non-lipid soluble substances.
impermeable
________ compounds dissolve in fat therefore they cross the phospholipid cellular membrane more easily.
lipophilic
lipophobic compounds are ______ therefore they cannot cross the phospholipid cellular membrane
ionized
what are the four methods of drugs and other substances to cross the membrane?
passive diffusion
facilitated diffusion
active transport
endocytosis
what factors does passive diffusion depend upon?
concentration, electrical, and pressure gradients
membrane permeability
temperature
what is diffusion trapping?
describes the inability of substances to pass the membrane because of its ion state; applies mostly as the body attempts to excrete the drug in urine
weak acids remain non-ionized in a/an _____ environment (like in the stomach) allowing it to be lipid soluble and be rapidly_____.
acidic; absorbed
weak bases remain non-ionized in a/an _____ environment (like in the duodenum) allowing it to be rapidly_____.
basic; absorbed
within the kidneys, where are weak bases and acids reabsorbed?
weak bases = absorbed proximally
weak acids = absorbed distally
T or F: substances which are ionized in a basic environment become trapped inside the nephron and are ultimately excreted in the urine.
F; acidic
what is a tight junction?
a junction formed where the outer leaflets of the opposed cell membranes made such close contact that the extracellular space between the adjacent cells is occluded
ex: blood-brain barrier
what must occur if a drug experiences a tight junction?
the drug must diffuse into and then out of the other side of the cells comprising the barrier
T or F: simple drugs and electrolytes can be carried through pores into a cell along the passive diffusion of water via osmosis.
T
what are the characteristics that comprise active transportation?
must include a carrier
must hace specificity/ affinity
requires energy expenditure in the form of ATP
can transport against a concentration gradient
T or F: specificity is not absolute, and some compounds that resemble another may be transported by the same group of carriers.
T
what is endocytosis (and exocytosis)?
the engulfment and regurgitation of substances by the cell wall allowing large nonlipid-soluble drugs to enter the cell under very specific circumstances
name a few locations where drugs are distributed within the body.
fat, tissue, targeted tissue, plasma protein, etc.
what are the four dependents of drug distribution?
tissue permeability: lipid soluble drugs readily enter lipid tissues
blood flow: higher blood flow enhances drug delivery
binding to plasma proteins: protein bound drugs diminished with protein deficiency
binding to sub-cellular components: certain drugs require sub-cellular components to operate
what is the volume of distribution equation?
Vd = dose / [plasma]
Vd unit is in liters!
the less amount of drug in the blood, the ____ it is distributed elsewhere.
more
a volume distribution of 42 L (medium amount) is indicative of…
uniform distribution
a volume of distribution of 8 L (small amount) is indicative of…
retainment in plasma
a volume of distribution of 420 L (large amount) is indicative of…
sequestration in tissues