5.2 Factors Affecting Rate of Reactions
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
Reaction Rate
The change in concentration of a reactant or a product of a chemical reaction per unit time
What are the five factors that affect the rate of the reaction?
Chemical Nature of Reactants
Concentration of Reactants
Surface Area
Temperature of the Reaction System
Presence of a Catalyst
Chemical Nature of Reactants
The chemical nature of a pure substance determines its chemical properties.
A chemical property describes how a pure substance behaves during a chemical change or reaction.
Examples of chemical properties include a substance's tendency to undergo combustion or oxidation reactions.
Chemical properties can strongly influence the reaction rate.
Oxidation reactions demonstrate differences among metals:
Sodium and potassium are highly reactive with oxygen and other substances, reacting so quickly they are never found naturally in their pure elemental form.
Platinum, gold, and silver are generally unreactive, and oxidation occurs very slowly.
Unreactive metals like platinum, gold, and silver are ideal for jewellery and electronics due to their resistance to oxidation.

Concentration of Reactants
Hospitals warn against smoking or flames near oxygen because:
Normal air is about 20% oxygen.
Medical oxygen tanks can be 50% oxygen or higher.
Higher oxygen concentrations cause combustion to be rapid, violent, or explosive.
Reaction rates often increase with higher reactant concentrations.
Example: Zinc reacts with hydrochloric acid to form zinc chloride and hydrogen gas:
Equation: Zn(s) + 2 HCl(aq) → ZnCl₂(aq) + H₂(g)
If you place zinc in increasingly concentrated hydrochloric acid:
Reaction is slow in dilute acid.
Reaction rate increases as acid concentration increases.

Surface Area
Reaction rate can be increased by increasing the surface area of a solid reactant.
Example: Cutting zinc into smaller pieces speeds up its reaction with hydrochloric acid.
Similar to how powdered sugar dissolves faster in water than sugar cubes.
In reactions involving solids and liquids, more surface area leads to a faster reaction.
Only atoms or ions on the surface of a solid can react.
Increasing surface area is like increasing concentration for solids.
Kindling burns faster than a log due to its higher surface area.
Fine flour dust can combust so quickly that it may cause explosions.
Workplaces handling fine particles (e.g., flour mills) must control dust to prevent fires and protect workers.
Temperature
Reaction rates increase with temperature.
Many reactions double in rate for every 10 °C increase, and halve for every 10 °C decrease.
Example: Cake batter changes very little at room temperature but undergoes reactions and bakes at 175 °C.
Refrigeration slows down chemical reactions that cause food spoilage.
Medications often include instructions to store in cool places to slow unwanted reactions.
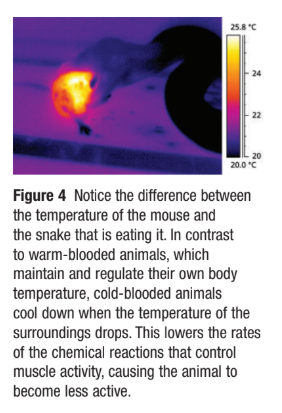
Cold-blooded animals (e.g. snakes, reptiles):
Take on the temperature of their surroundings.
Are less active in cooler conditions due to slower metabolic rates.
Infrared images show cold-blooded animals as cooler colors (blue/black) and warm-blooded animals as warmer colors (yellow/orange).
Catalysts
A catalyst speeds up a chemical reaction but remains unchanged after the reaction.
Can be reused many times.
Only small amounts are typically needed.
Biological Catalysts (Enzymes):
Enzymes are protein molecules that act as biocatalysts.
Enable reactions in cells to occur at moderate temperatures.
Example: Lactase helps digest lactose in milk.
Adults often produce less lactase → lactose intolerance.
Used in the production of:
Beer, cheese, yogurt, medicines, and enzyme-based detergents.
Most enzymes are made via fermentation using bacteria, yeast, or moulds.
Enzymes are sensitive to temperature and pH, so research focuses on making them more stable for industrial use.
Benefits of catalysts:
Speed up reactions.
Lower required temperatures → less energy used, less environmental harm.