CHEMISTRY TOPIC 2- Bonding, Structure, and Properties of Matter
1/29
Earn XP
Description and Tags
Bonding, Structure, and Properties of Matter
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Ions and How they’re Formed
Charged particles
Formed when elements lose or gain electrons to gain a full outer shell
Electrons are transferred to other element in ionic bonding to fill their outer shell also
Full outer shell = very stable and inert
When forming ions, the quickest/easiest method of making a full outer shell will be used e.g. group 6 elements could lose 6 electrons or just gain 2
Cations and Anions
Cations are positive ions
Anions are negative ions
How Metals form Ions
Metals lose electrons from outer shell to form cations (positive)
How Non-metals form Ions
Non-metals gain electrons to outer shell to form anions (negative)
Groups most Likely to form Ions
Groups 1, 2, 6, 7
They need to gain/lose few electrons to achieve a full outer shell
Charge of an Ion in Group 1
Group 1 elements have 1 electron in outer shell, so they need to lose one electron to form an ion
This means they are misusing 1 negative charge (- - 1)
-- - 1 = +1 , so group 1 elements form 1+ ions
Charge of an Ion in Group 6
Group 6 elements have 6 electrons in outer shell, so need to gain 2 electrons to form an ion
This means they are adding 2 negative charge (+ - 2)
+ - 2 = -2 , so group 6 elements form 2- ions
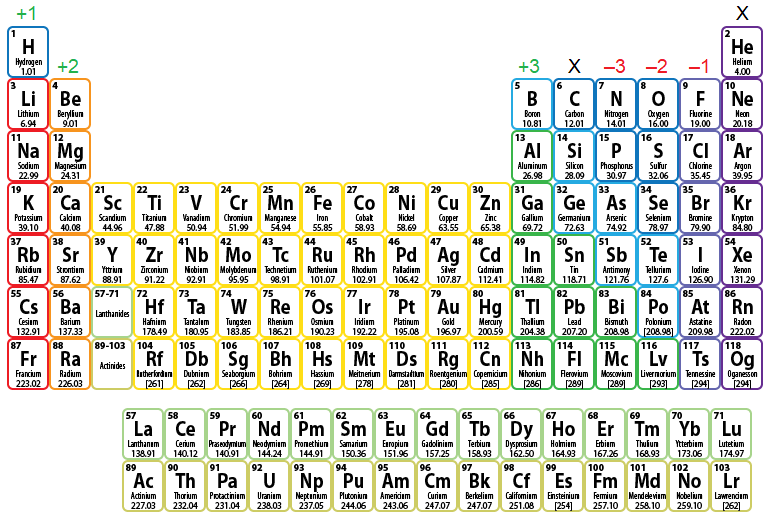
Ionic Bonding
Transfer electrons between elements to give both full outer shells
Between metal and non-metal
Metal atoms transfer ions to non-metals
Strong intermolecular forces
Oppositely charged ions provide strong electrostatic forces
Ionic Compound Structure
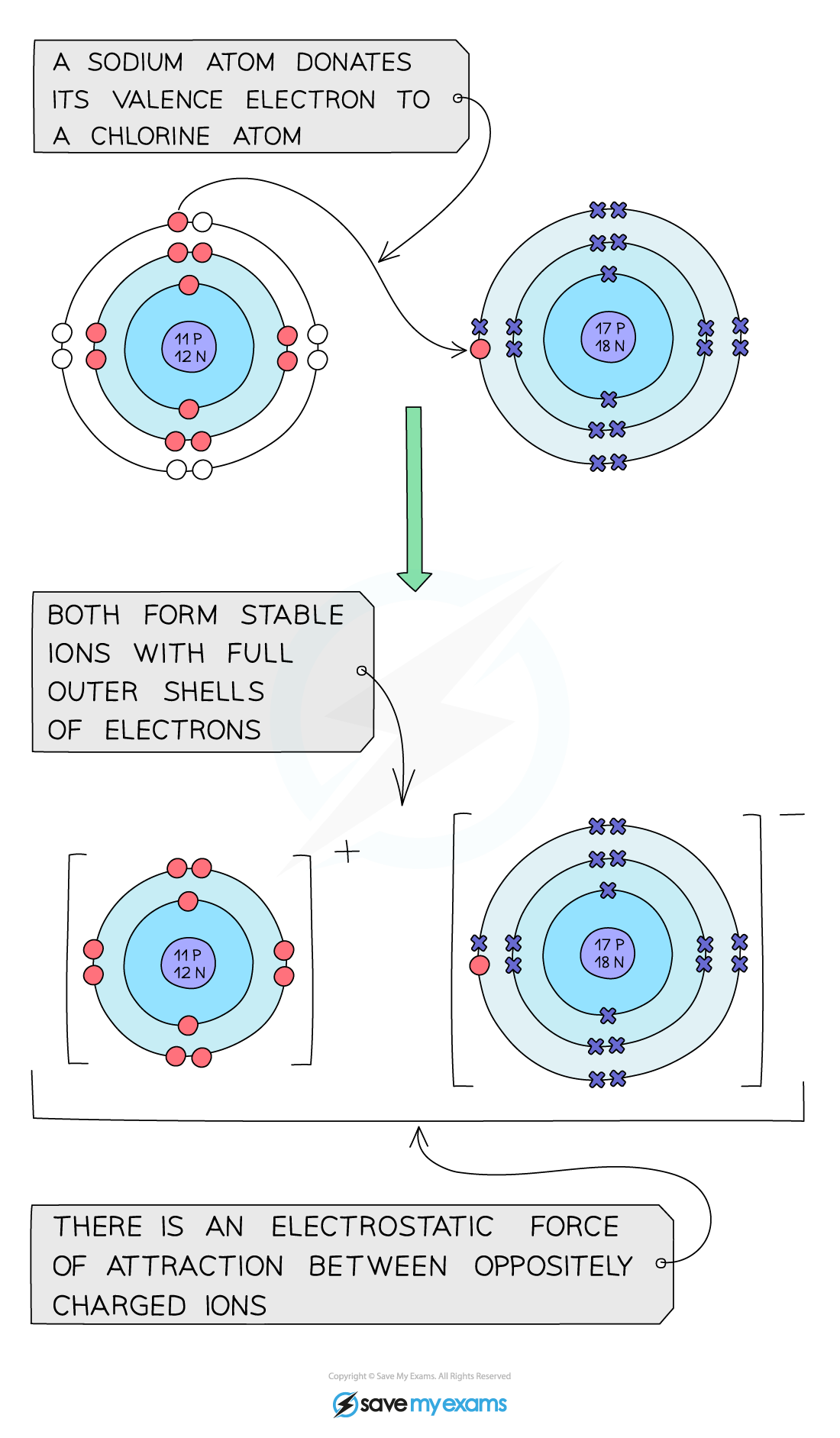
Giant Ionic Lattice Structure
Regular arrangement of ions held together by strong electrostatic forces and ionic bonds
Ionic Compound Properties
High melting and boiling points due to strong ionic bonds
Only conduct electricity when molten or dissolved in solution (ions cannot move and carry a current when solid)
Strong intermolecular forces
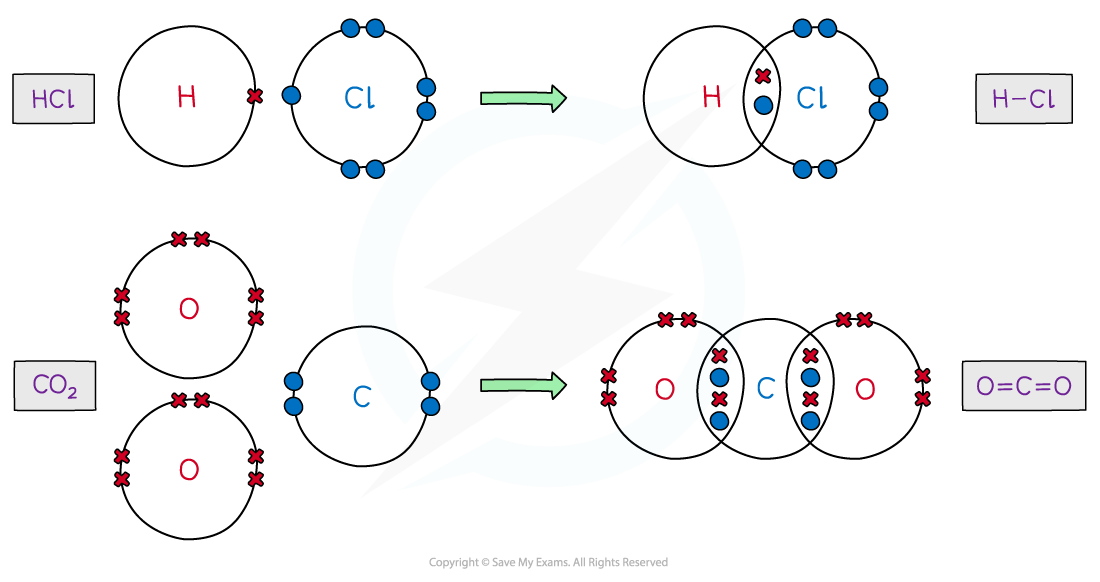
Covalent Bonding
Share electrons between elements to give both full outer shells
Between non-metal and non-metal
Very strong covalent bonds- positive nucleus attracted to shared pair of electrons by electrostatic forces
Only outer shell electrons are shared
These bonds can form single, double, or triple bonds depending on the number of shared electron pairs e.g. one pair of electrons shared = one bond
Ways to Draw Covalent Bonds
Dot and Cross diagrams
Displayed Formula e.g. H — Cl
3D Model
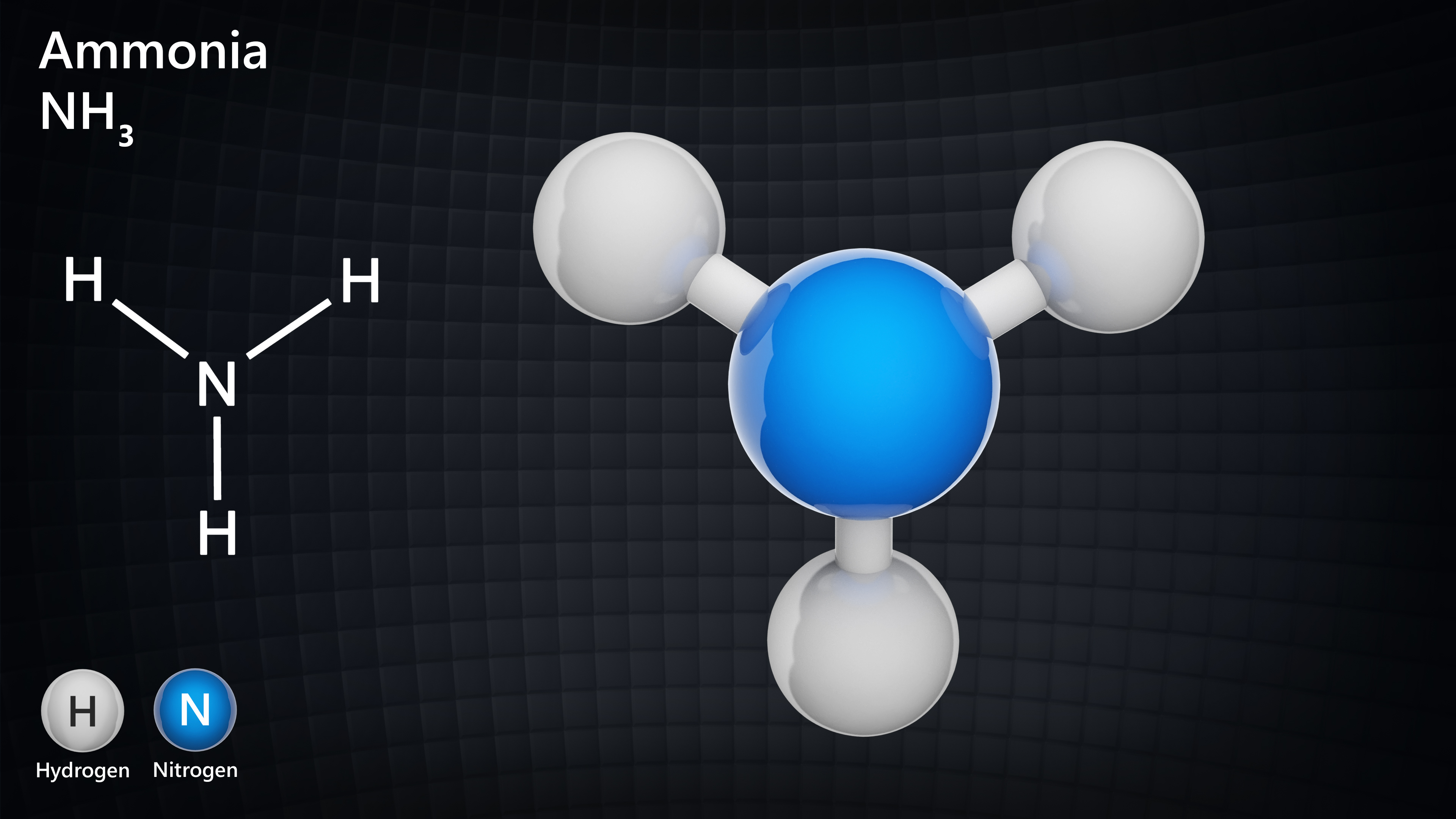
Examples of Simple Molecular Substances
All have simple single covalent bonds
Hydrogen, H2
Chlorine, Cl2
Oxygen, O2
Nitrogen, N2
Methane, CH4
Water, H2O
Hydrogen Chloride, HCl
Carbon Dioxide, CO2
Simple Molecular Substance Properties
Contain very strong covalent bonds
Contain very weak forces of attraction ( same charge molecules)
Low boiling points usually- only need to break week intermolecular forces, not the strong covalent bonds
As molecules get bigger, intermolecular forces increase (boiling point increases since more energy need to break forces)
Most are gas/liquid at room temperature
Don’t conduct electricity- no free electrons/ions
Polymers
Long chain of repeating units (monomers)
Atoms joined by covalent bonds
Molecular formula for polymer = (repeating unit)n where n is the number of repeats
Large intermolecular forces- most are solid at room temperature and high boiling points
Lower boiling points than ionic and giant molecular compounds
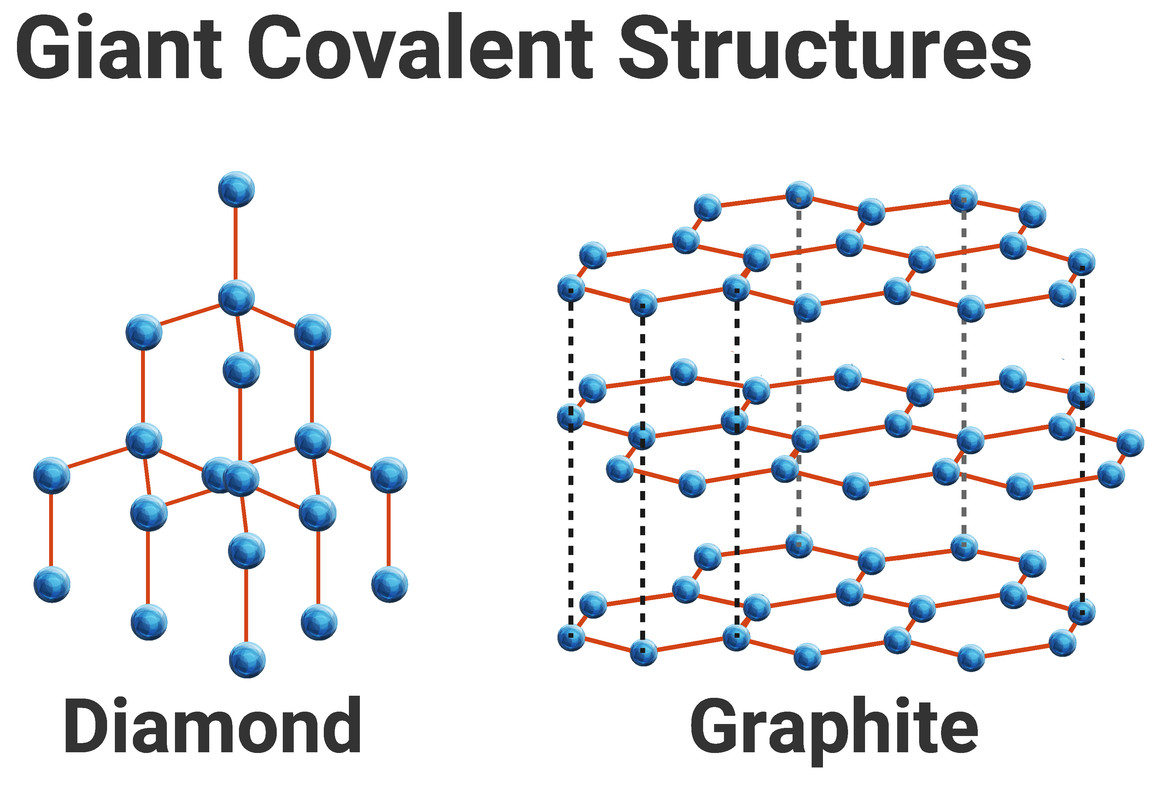
Giant Covalent Structures / Macromolecules
Atoms bonded by strong covalent bonds
Very high melting and boiling points- lots of energy required to break bonds
Don’t conduct electricity (except for exceptions e.g. graphite with delocalised electrons)
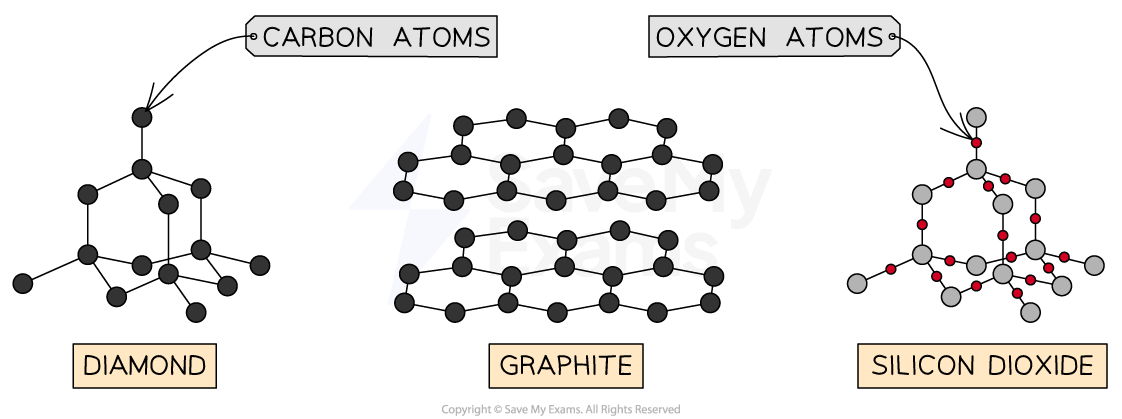
Giant Covalent Bonds Examples
Diamond- each carbon atom has 4 covalent bonds
Graphite- each carbon forms 3 covalent bonds, made of layers of graphene which can slide over one another due to weak intermolecular forces; also conductive (each carbon has 1 delocalised electron)
Silicon Dioxide (Silica) - sand
Allotropes of Carbon
Different structures of carbon giving varying properties in the same state
Diamond- very hard due to each carbon having 4 covalent bonds, high melting point, nonconductive
Graphene- layer of graphite, sheet of carbon atoms joined to make hexagons, light, strong, conductive due to delocalised electrons from carbon
Graphite- each carbon has 3 covalent bonds, organised in sheets of graphene made of hexagons, conductive due to delocalised electrons meaning it can be used in electronics
Fullerene- structured in ball-like or tube shapes; arranged in pentagons, hexagons, or heptagons. Can be used to cage molecules and deliver drugs to body. Large surface area so useful for industrial catalysts. Can be used as lubricant. Form nanotubes (conductive, high tensile strength, used in electronics or strengthening without adding weight)
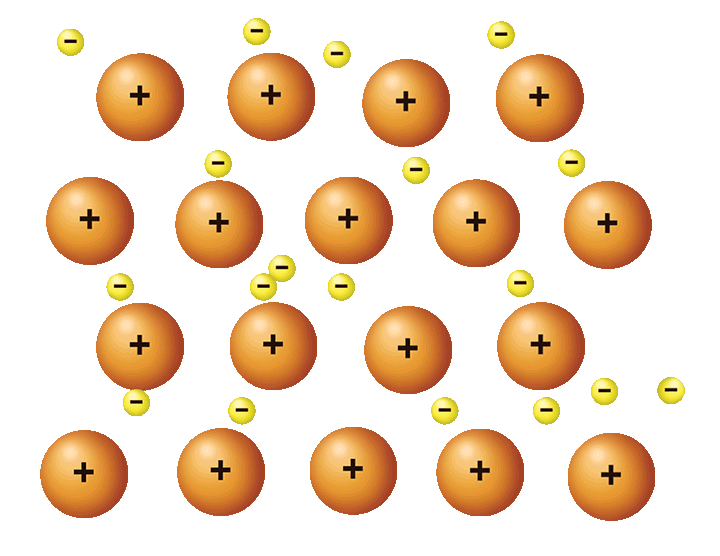
Metallic Bonding
Positively charged metals bond in a sea of their delocalised electrons keeping them bonded due to the electrostatic forces between the negative electrons and positive metal atoms/ions
Between metal and metal
Outer shell electrons delocalised, meaning high conductivity and strong electrostatic forces
Properties of most Metals
Solid at room temp
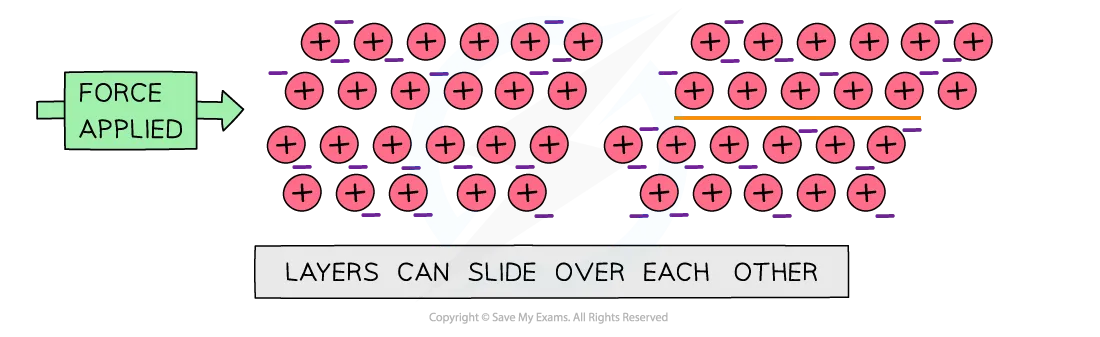
Malleable (bendable)- metal layers can slide over one another
Good electric and thermal conductors
Ductile (can be drawn into wires)
High melting and boiling points
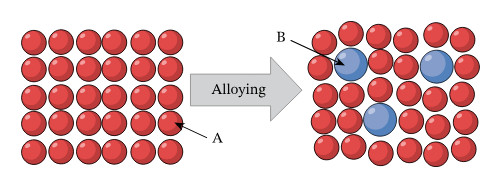
Alloys
Mixture of metals
Better stability
Atoms in alloy have different sized atoms, meaning the layers are distorted so they can’t slide over each other as easy (harder than pure metals)
Higher boiling points
Solids
State symbol (s)
Strong forces of attraction between particles
Forces hold particles in fixed positions in regular lattice arrangement
Keep definite volume and shape
Particles vibrate in fixed positions (heat increases vibrations, causing expansion)
Liquids
State symbol (l)
Weak forces of attraction between particles
Random arrangement, free to move past one another but stay close together
No definite shape/volume- liquids flow to fill bottom of container
Constant movement with random motion (heat increases speed, leads to expansion)
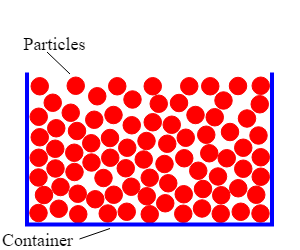
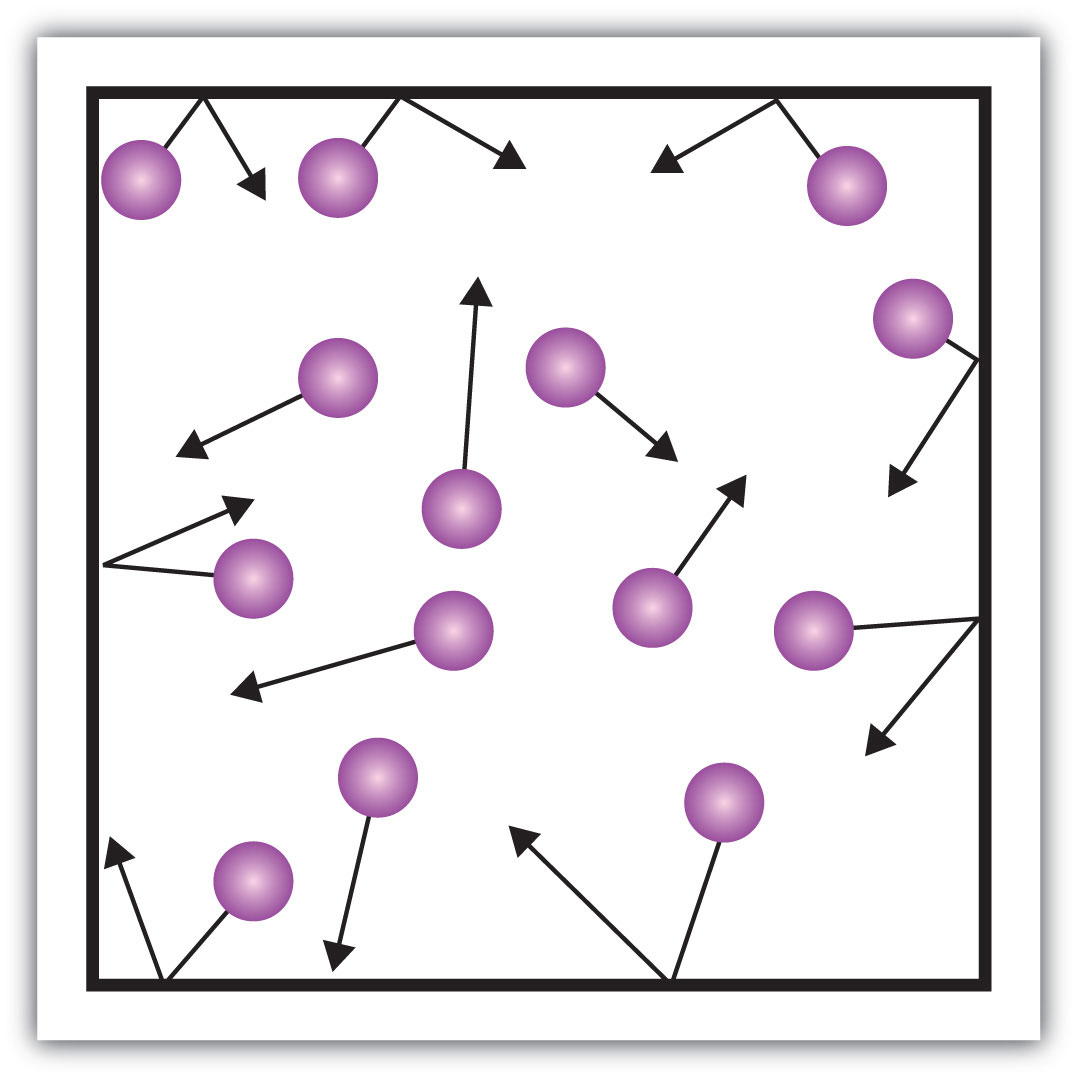
Gases
State symbol (g)
Very weak forces of attraction between particles
No fixed arrangement, particles free to move
Particles travel in straight lines
No definite shape or volume, always fills container
Constant random movement (heat increases movement speed leading to further expansion)
Heat and pressure increase lead to gas expanding
Changing State: Solid → Liquid
Melting
Changing State: Liquid → Solid
Freezing
Changing State: Liquid → Gas
Boiling / Evaporation
Changing State: Gas → Liquid
Condensing
Changing State: Solid → Gas
Sublimation
Changing State: Gas → Solid
Deposition