Moles
1/14
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Mole
Avogadro's number of an atom, ion or compound
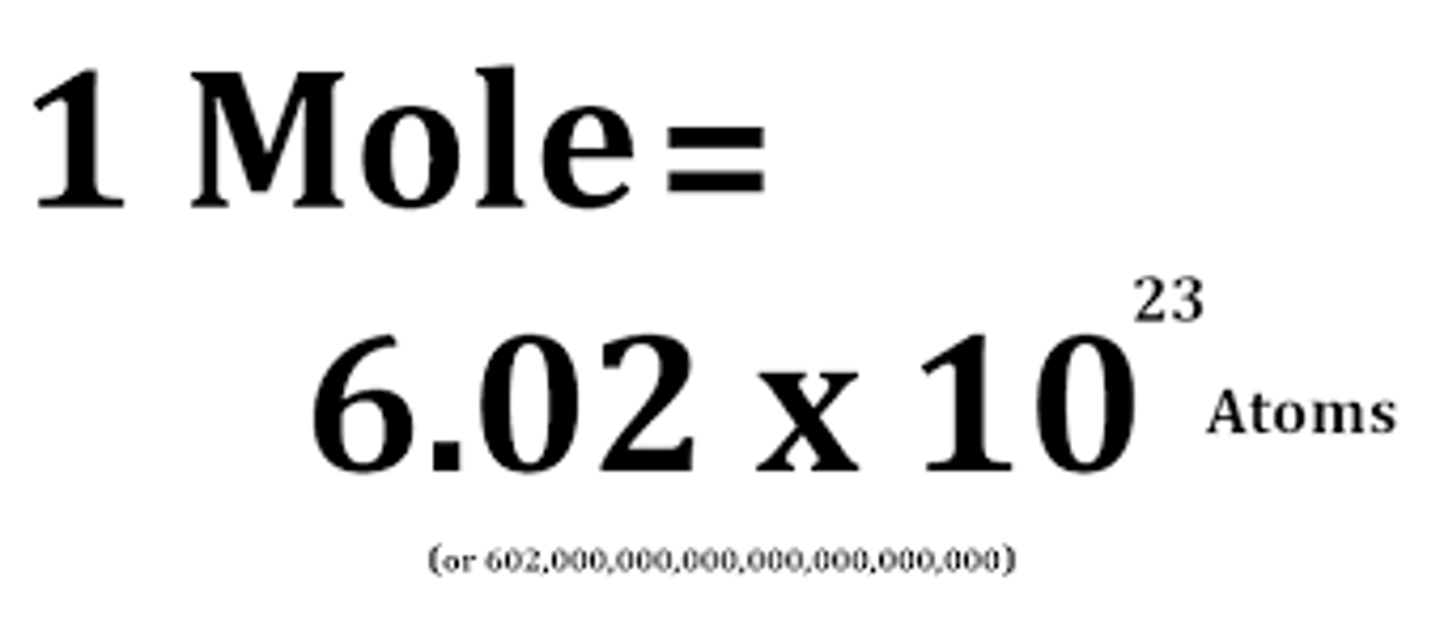
Formula for moles
Mass(g)/relative formula mass (Mr)
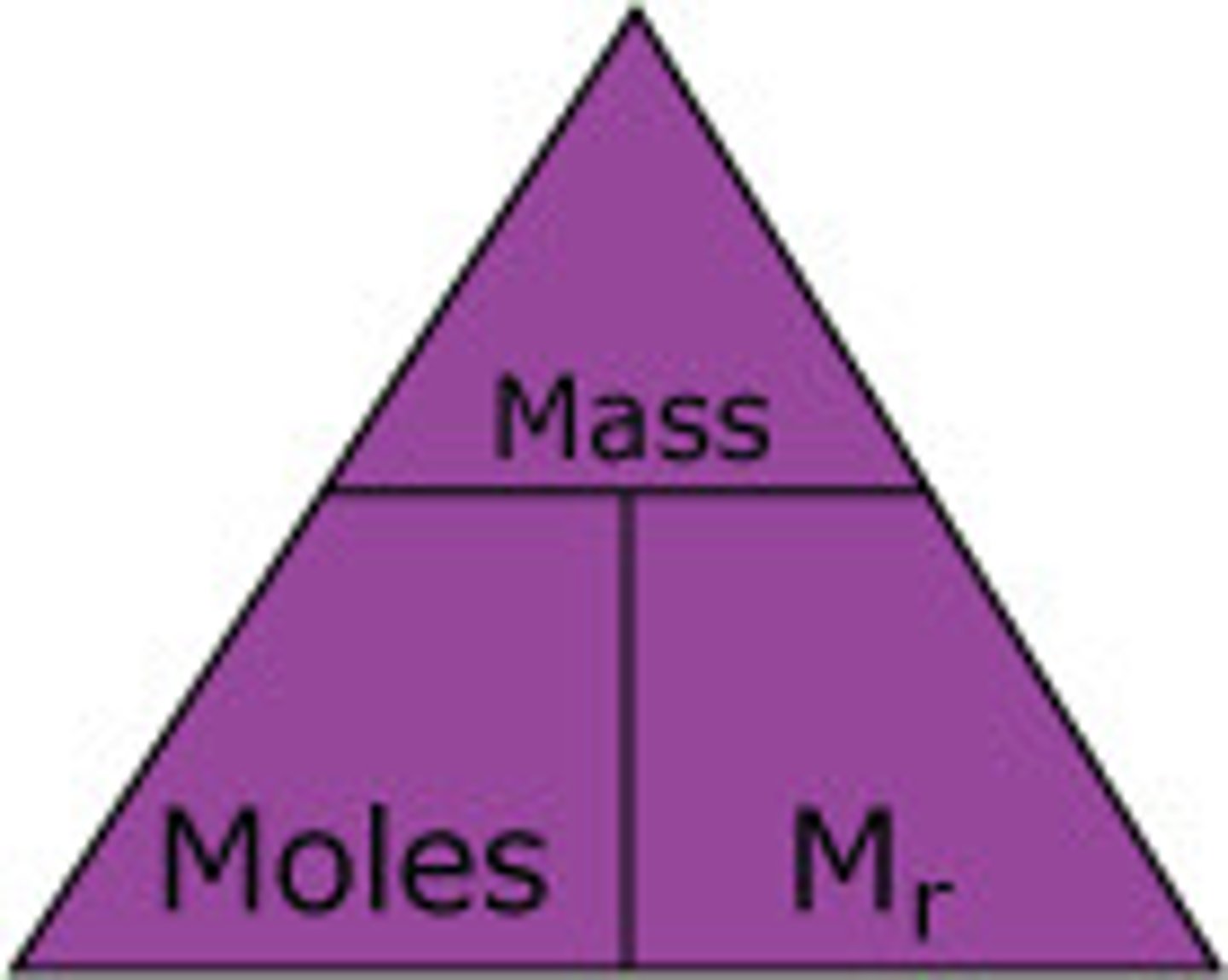
The mass of 1 mole of any substance=
formula mass in grams
mol
Symbol for moles
Value of Avogadro constant
6.02 x 10^23

Avogadro's constant
The number of atoms, molecules or ions in a mole of a given substance
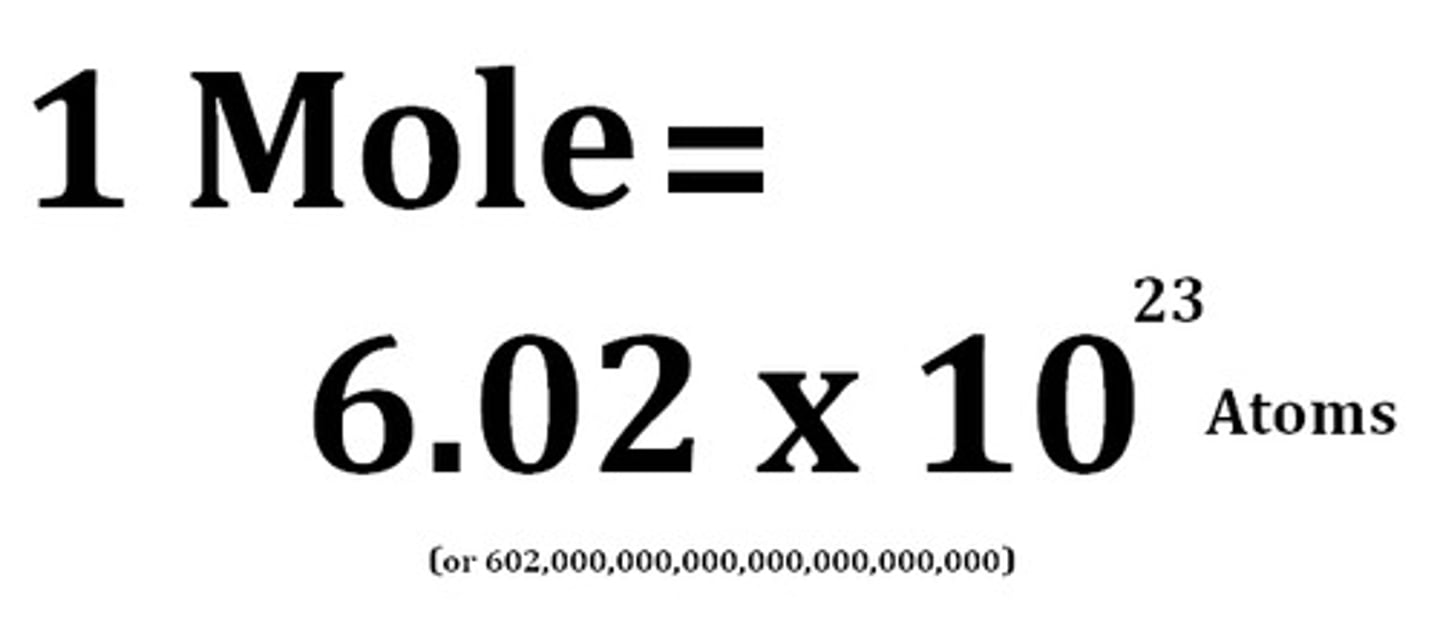
Relative atomic mass
The weighted mean mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12.
Relative formula mass
The relative atomic masses of the elements in a compound added together
Reacting mass
The amount of one reactant needed to react exactly with another reactant.
Link between moles and equations
Multipliers show you the ratio of moles in a reaction
Reagent
Reactant
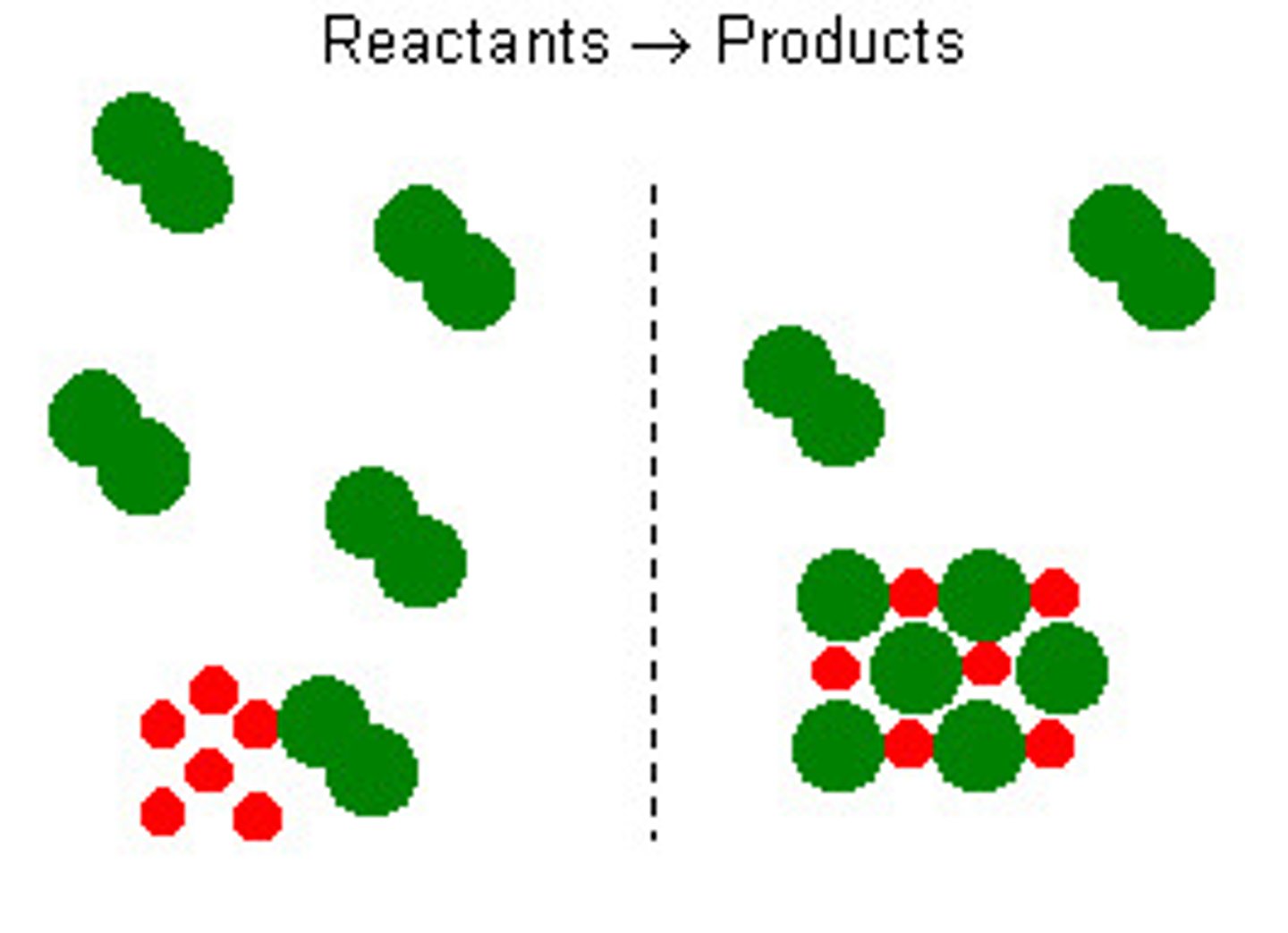
Limiting reactant (limiting reagent)
A reactant that is totally used up so it determines the amount of product formed.
Excess
More than is required to react with the limiting reactant
1 kg
1000 g
1 tonne
1000 kg