12.2 Ideal gas law
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
30/5/22
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
universal gas constant
R = 8.314 kPaL/Kmol (kilopascal litres/ Kelvin mol)
2
New cards
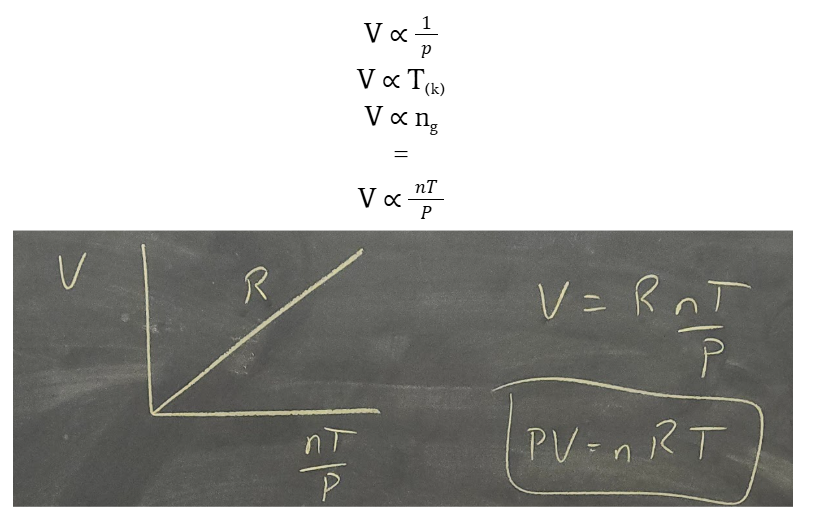
ideal gas law
R = PV / nT or PV = nRT
3
New cards
use ideal gas law to find molar volume
PV = nRT
V = nRT/P
V/n = RT/P
since V/n is molar volume, mV = RT / P
4
New cards
use ideal gas law to find density
D = m/V
V = m/D
substitute V = m/D into PV = nRT
Pm/d = nRT
Pm = nRTD
D = Pm/nRT
since m/n is mm, D = mmP/RT
since P/RT is mV, this is the same as D = mm/mV
5
New cards
use ideal gas law to find mm
substitute n = m/mm into PV = nRT
PV = mRT/mm
mmPV = mRT
mm = mRT/PV