PHAR 230 - TEST 2 - Modules 2 & 3
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
99 Terms
Break down the nervous system.
CNS & PNS
PNS: Nerves & Sensory receptors
Nerves: Afferent division (sensory) & Efferent division (motor)
Efferent division: autonomic & somatic
Autonomic: sympathetic & parasympathetic
Sympathetic: fight/fight
Parasympathetic: rest/digest
Describe the characteristics of the sympathetic and parasympathetic nervous systems.
Sympathetic: CNS neuron located in lateral gray horns of T1-L2; PNS ganglia near vertebral column; preganglionic fibers short - releasing acetylcholine; postganglionic fibers long - releasing norepinephrine
Parasympathetic: CNS neuron located in brain stem and S2-S4; PNS ganglia within vertebral column; preganglionic fibers long -releasing acetylcholine; postganglionic fibers short - releasing acetylcholine
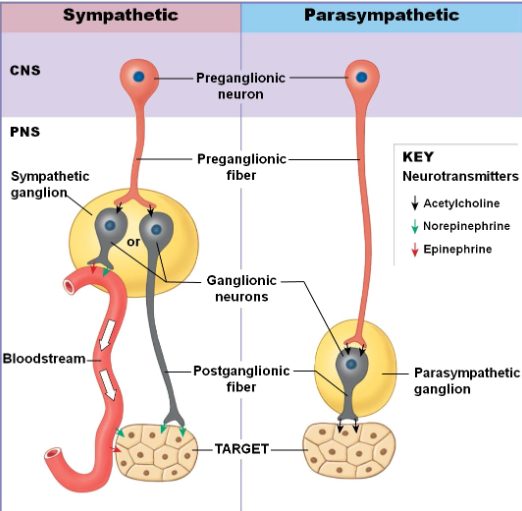
What are the 3 types of synapses in the sympathetic nervous system and list their neurotransmitter, location, effect, and fate.
Cholinergic synapses:
Release acetylcholine
Located in sympathetic ganglion (where preganglionic neurons synapse with postganglionic neurons) or postganglionic fibers of sweat glands
Effect: localized, always excitatory, persists for 20 msec
Fate of neurotransmitter: broken down by enzymes:
acetylcholinesterase at synapses
pseudocholinesterase in surrounding tissue
Adrenergic synapses:
Release norepinephrine
Location: postganglionic fibers synapse with effectors (target muscle/gland, sometimes organ). This excludes sweat glands, which are cholinergic.
Effect: localized; excitatory or inhibitory; persists for few seconds
Fate of neurotransmitter:
Reuptake: 50-80% transported back to presynaptic neuron to be reused or broken down by monoamine oxidase (MAO) enzyme.
Broken down by catecholamine-O-methyltransferase (COMT) enzyme in synaptic cleft
Adrenal medulla transmitters:
Release 80% epinephrine & 20% norepinephrine
Location: whole body
Effect: generalized; act as hormones; carried by bloodstream throughout body
Fate: lasts longer as blood does not contain MAO or COMT
What are adrenergic receptors?
Protein molecules in target organs.
2 Classes: alpha receptors (a1 & a2); beta receptors (b1,b2,b3)
What adrenergic receptors do epinephrine and norepinephrine stimulate?
Epinephrine: all alpha & beta receptors
Norepinephrine: all alpha receptors, b1, and b3 (NOT b2)
Describe a1 receptors.
More abundant than a2
Excitatory effect on target cell:
Vasoconstriction of blood vessel
Closure of sphincters
pupil dilation
Describe a2 receptors.
Inhibitory effect on target cell
Decreases release of norepinephrine
Help coordinate sympathetic and parasympathetic activities.
Describe the effects of all the beta receptors.
b1:
Excitatory effects:
Inc smooth muscle contraction
Inc heart rate & contractility
Inc blood pressure
b2:
Inhibitory effects:
Dec respiratory smooth muscle contraction → bronchodilation
Dec smooth muscle contraction → vasodilation & relaxation of walls
b3:
In adipose tissue
Stimulation → lipolysis
Where are a1,a2,b1, and b2 receptors located?
a1: smooth muscles (most tissues)
a2: CNS
b1: heart
b2: lungs, blood vessels, GI muscle, urinary muscles, uterus
What is the neurotransmitter of the parasympathetic nervous system?
Acetylcholine: broken down by acetylcholinesterase at synapse and pseudocholinesterase in surrounding tissue
Describe the 2 types of cholinergic receptors.
Nicotinic:
Sensitive to nicotine
Location:
Neuronal nicotinic receptors (Nn): autonomic ganglion cells
Neuromuscular nicotinic receptors (Nm): neuromuscular junctions of skeletal muscles
Always excitatory
Muscarinic:
Sensitive to muscarine
Location: cholinergic neuromuscular or neuroglandular parasympathetic junctions; cholinergic sympathetic junctions (sweat glands)
Effects are longer lasting than nicotinic receptors
Excitatory or inhibitory
What two drugs act on the sympathetic nervous system?
Sympathomimetic agents: adrenergic drugs; stimulate SNS; agonists
Sympatholytic agents: antiadrenergic drugs; inhibit SNS; antagonists
What are the 3 classes of sympathomimetic agents? Describe them.
Direct acting: bind directly to receptors and elicit a response (e.g., epinephrine)
Indirect-acting: increase release of norepinephrine from presynaptic neuron (e.g., amphetamines)
Mixed: both direct & indirect effect (e.g., ephedrine)
What are the therapeutic indications for anaphylaxis, heart, asthma, nasal congestion and pupil dilation in relation to sympathomimetics?
Anaphylaxis - a & b agonists
Heart - b1 agonists
Asthma - B2 agonists
Nasal congestion - a1 agonists
Pupil dilation - a1 agonists
What are the 3 classes of sympatholytic agents?
a receptor antagonists: non-selective, a1 blockers, a2 blockers
b receptor antagonists: non-selective, b1 blockers, b2 blockers
a and b receptor antagonists
What are the therapeutic indications of hypertension, angina/CHF, dysrhythmias, and impaired peripheral circulation (raynaud’s) in relation to sympatholytic agents?
Hypertension - b blockers (preferably b1 blockers); a & b blockers during pregnancy
Angina/CHF - selective b1 blockers
Dysrhythmias: selective b1 blockers
Impaired peripheral circulation (Raynaud’s): a1 blockers
What are the therapeutic indications of pheochromocytoma in relation to sympatholytic agents?
a & b blockers
What are the therapeutic indications of benign prostatic hyperplasia, glaucoma, and neurological disorders in relation to sympatholytic agents?
BPH: a1 blockers
Glaucoma: b blockers
Neurological disorders: b blockers
What are the adverse effects of a-blockers?
Orthostatic hypotension
Nasal congestion
Headache, dizziness, weakness, fatigue
What are the adverse effects of b blockers?
Bronchoconstriction
Bradycardia, hypotension, exacerbation of heart failure, cold extremities
Depression, nightmares, hallucination, paresthesia
Masking the effects of hypoglycemia.
What are the adverse effects of a and b agonists?
Headache, restlessness, tremors, dizziness, anxiety, insomnia
Dysrhythmia, palpitation, vasoconstriction, hypertension, myocardial ischemia
Anorexia, nausea, vomiting, dry mouth, muscle cramps.
What 2 types of drugs act on the parasympathetic nervous system?
Parasympathomimetic agents:
Cholinergics, cholinomimetics, cholinoreceptor activating drugs
Stimulate PSNS
Parasympatholytic agents:
Anticholinergics, antiparasympathomimetics
Inhibit PSNS
How are parasympathomimetic agents used?
To stimulate muscarinic receptors or nicotinic receptors.
Not widely used as they can cause bradycardia and bronchoconstriction.
Used in treatment of:
Glaucoma
Muscarinic receptor stimulants increase ciliary body contraction, increasing fluid drainage from eye, decreasing IOP
Myasthenia gravis
poor muscle tone in bladder
Ileus
Used in diagnosis of asthma.
How is glaucoma treated?
Glaucoma, increased IOP due to fluid, is treated by increasing fluid outflow and reducing fluid secretion.
Increasing fluid outflow is done with:
Prostaglandin analogues
Cholinomimetics (a parasympathomimetic agent; Muscarinic agonists)
Reduced fluid secretion is done with:
Beta blockers
Carbonic anhydrase inhibitors
Alpha agonists are also used to simultaneously increase fluid outflow and reduce fluid secretion.
How are parasympathomimetic agents used to diagnose asthma?
Administering methacholine, a muscarinic agonist, causing bronchoconstriction. If there is a 20% drop of pulmonary function, person diagnosed with asthma.
What are the 2 classes of parasympatholytic agents?
Antinicotinic agents (ganglion blockers): block N receptors
Antimuscarinic agents (muscarinic blockers): block M receptors
Describe antinicotinic agents (ganglion blockers)
Block autonomic ganglia, inhibiting both SNS & PSNS
Limited therapeutic use due to wide range of adverse effects
Describe Atropine.
A muscarinic receptor blocker
Competitive antagonist of all M receptors
strong, long lasting anticholinergic effect
Causes dilated pupils, tachycardia, bronchodilation, inhibits secretions
Describe organophosphate compounds and poisoning.
Irreversibly inhibit acetylcholinesterase (ACHE) by phosphorylating serine residue on active site.
E.g., nerve gases (sarin) and insecticides (malathion)
Poisoning manifests as DUMMBBLESS:
Diarrhea
Urination
Miosis
Muscle spasm
Bradycardia
Bronchoconstriction
Lacrimation
Emesis
Salivation
Sweating
Poisoning treated with atropine and oximes.
What groups of drugs have similar but not identical effects?
Sympathomimetic and parasympatholytic drugs: fight/flight
Sympatholytic and parasympathomimetic drugs: rest/digest
Where does somatic motor nervous system act?
Act at neuromuscular junction (NMJ):
Synapse between a synapatic terminal of neuron & motor end plate of skeletal muscle fiber
Neurotransmitter is acetylcholine which acts on nicotinic (Nm) receptors to produce muscle contraction
What are the 2 classes of neuromuscular blocking drugs?
Non-depolarizing:
Competitive antagonists to acetylcholine
Block nicotinic Nm receptors at neuromuscular junction
This prevents muscle depolarization and action potential
This prevents muscle contraction, causing muscle paralysis
Can be overcome by using acetylcholinesterase inhibitors
Depolarizing:
Acetylcholine agonists
Activate nicotinic Nm receptors at neuromuscular junction
This causes muscle contraction
Not inhibited by acetylcholinesterase enzyme
This causes continuous and prolonged activation of Nm receptors
This causes persistent muscle depolarization
This results in no time allowed for repolarization and relaxation
This causes muscle exhaustion and paralysis
What are the 2 phases of depolarizing neuromuscular blockers?
Depolarizing phase:
Disorganized depolarization of muscle fibers
Twitching
unable to repolarize
Desensitizing phase:
After prolonged exposure to drug
Causes muscle repolarization and no longer responsive to acetylcholine
This causes muscle to become desensitized
Results in flaccid paralysis
Paralysis prolonged by using acetylcholinesterase inhibitors
What are the adverse effects of neuromuscular blockers?
Paralysis of diaphragm and respiratory failure
Depolarizing blockers:
Hyperkalemia
Muscle pain
Malignant hyperthermia
When do you increase and decrease neuromuscular blocker dose?
Increase dose for burns and upper motor neuron lesion.
Decrease dose for old age and myasthenia gravis.
How does botulinum toxin act on NMJ?
Blocks release of acetylcholine, stopping muscle contraction
What are the 5 major classes of diuretics?
Thiazide diuretics (K+ losing; calcium sparing)
Loop diuretics (K+ losing; calcium losing)
Potassium sparing diuretics (K+ sparing; Calcium sparing)
Carbonic anhydrase inhibitors (K+ losing)
Osmotic diuretics
Describe thiazide diuretics.
Site of action: distal convoluted tubule.
Decreases NaCl cotransporter, decreasing NaCl reabsorption, resulting in water staying in the urine (less reuptake) = more urine
Gentle diuresis = safe in elderly
Adverse effects:
Hypokalemia (common)
Hypomagnesemia
Hyperglycemia, hypercalcemia, hypercholesterolemia, hyperuricemia
Describe loop diuretics.
Site of action: thick segment of ascending loop of Henle
Decreases Na-K-Cl cotransporter, decreasing NaCl reabsorption, resulting in water staying in urine and increased Ca & Mg excretion.
Strong diuretic
Adverse effects similar to thiazides except:
Hypocalcemia → osteoporosis
Ototoxicity → deafness
Describe potassium sparing diuretics
2 Classes:
Aldosterone antagonists:
Competitive inhibition of aldosterone at receptor site, decreasing Na and water reabsorption while reabsorbing K.
Sodium channel blockers:
Decreased Na reabsorption → water stays in urine
Weak diuretics
Site of action: Distal convoluted tubule and collecting ducts
NaCl and water loss in urine; saves K+ (potassium sparing)
Adverse effects:
Hyperkalemia (common)
Spironolactone: androgen or estrogen like effects
Describe carbonic anhydrase inhibitors.
Site of action: proximal convoluted tubule
Decreases carbonic anhydrase enzyme, decreasing H+ excretion, increasing Na+ and K+ excretion.
Decreases intraocular pressure (treatment of glaucoma)
Adverse effects:
Metabolic acidosis
Hypokalemia
Describe osmotic diuretics.
Site of action: throughout nephron, especially proximal convoluted tubule
Filtered in glomeruli and cannot be reabsorbed from renal tubules, remain in tubular lumen, increasing osmolarity of tubular fluid, retaining water in urine
Decreases intracranial and intraocular pressure
Adverse effects:
Increases extracellular fluid volume
May aggravate heart failure and pulmonary edema
Describe diuretic resistance.
Decreased glomerular filtration rate due to low cardiac output (e.g., heart failure)
Increased proximal reabsorption of Na+
Decreased secretion into tubule lumen
Non-compliance
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Activation of renin-angiotensin-aldosterone system (RAAS)
How does sympathetic nervous system activity effect blood pressure?
Increased SNS = increased noradrenaline = vasoconstriction = increased BP
Vise versa
How does the renin-angiotensin-aldosterone system (RAAS) effect blood pressure?
Blood pressure falls, renin released, triggers angiotensin, triggers antidiuretic hormon release, increases salt and water reabsorption, increasing BP
What are the 2 types of hypertension?
Primary hypertension:
No identifiable cause
Most common
Secondary hypertension:
Caused by underlying disease:
Renal disorders
Endocrine disorders
Pregnancy
etc
What are the 5 types of antihypertensive drugs?
Diuretics
Sympatholytics
Calcium channel blockers
Drugs acting on RAAS
Vasodilators
What diuretic is most used to treat hypertension?
Thiazide diuretics
Describe sympatholytics in relation to hypertension
Includes a blockers, b blockers, a & b blockers, adrenergic neuron blockers, centrally acting drugs
a blockers:
Black a receptors, decreasing constricting effect of norepinephrine on smooth muscle cells of blood vessels, causing vasodilation
b blockers:
Widely used, cardioprotective
Block b receptors, decreasing sympathetic nervous system effect on cardiovascular system
Decreases HR, myocardial contractility, cardiac output, myocardial O2 demand, renin secretion
Can cause bronchospasm (asthma), bradycardia (heart block), mask hypoglycemia
Better avoid in elderly
Two types:
Nonselective b antagonist
Selective b1 antagonist (cardioselective, preferred)
a & b blockers:
Used in pregnancy, e.g., labetalol
Adrenergic neuron blockers:
Used in pregnancy, e.g., methyldopa
What 3 types of drugs act on RAAS?
Angiotensin converting enzyme inhibitors (ACE inhibitors)
Angiotensin receptor blockers (ARB)
Renin inhibitors
Describe ACE inhibitors.
A drug acting on RAAS.
Inhibit angiotensin-converting enzyme (ACE), preventing the conversion of angiotensin I into II and preventing antidiuretic hormone production, resulting in lower BP
Safe in asthma
No impotence (erectile dysfunction)
Useful in diabetes
Causes dry cough, hyperkalemia, teratogenic
Describe angiotensin receptor blockers.
Block the action of angiotensin II at the angiotensin 1 receptors
Similar effect as ACE inhibitors
Lower incidence of cough
What type of antihypertensive is recommended for:
Elderly & HF
Diabetes
Coronary artery disease
Pregnancy
Prostatic enlargement
Elderly & HF: diuretics
Diabetes: ACE inhibitors or angiotensin receptor blockers
Coronary artery disease: selective b1 blockers
Pregnancy: Methyldopa (Adrenergic neuron blocker) or labetalol (a & b blocker)
Prostatic enlargement: a blockers
AVOID:
b blockers in asthma and advanced HF
ACE inhibitors and angiotensin receptor blockers in pregnancy
What are the 2 types of angina?
Typical (exertional):
Chest pain due to exertion
Due to coronary obstruction
Variant (Prinzmetal’s):
Chest pain at rest
Due to coronary vasospasm.
What are the 3 types of antianginal drugs?
Organic nitrates (used in prevention/termination of angina attacks)
b blockers (used in long-term prophylaxis of typical angina, not variant)
Calcium channel blockers (used in long-term prophylaxis of variant angina, not typical)
Describe organic nitrates for treatment of angina.
Esters of nitric oxide
Short acting: glyceryl trinitrate (nitroglycerin/GTN)
Long acting: isosorbide dinitrate, isosorbide mononitrate
Nitrates reduced into nitro oxide, increasing guanlyl cyclase activity, converting GTP → cGMP, increasing PKG, decreasing intracellular calcium, and causing relaxation of smooth muscles of blood vessels - resulting in vasodilation.
Adverse effects:
Headache, flushing, hypotension, tachycardia, dizziness, methemoglobinemia, tolerance
Tolerance occurs due to continuous exposure; prevented with nitrate-free periods (overnight). Causes Monday disease.
Nitrates + PDE5 = severe hypotension (DDI)
What are the 3 forms of vasodilation?
Venous dilation:
Dec venous return
Dec cardiac output
Dec myocardial work
Dec myocardial O2 demand
Coronary dilation:
Inc blood supply to heart
Myocardial perfusion
O2 supply to ischemic myocardium
Arterial dilation:
Dec peripheral resitance
Dec blood pressure
Describe calcium channel blockers in relation to angina treatment.
Block L-type voltage gated Ca channels, preventing Ca flow into vascular smooth muscles - causing vasodilation in arteries rather than veins. Also prevents Ca flow into cardiomyocytes, decreasing cardiac contractility, decreasing CO
3 Classes:
Dihydropyridine: more vascular selective, e.g., nifedipine
Phenylalkylamine: more myocardial selective, e.g., verapamil
Benzothiazepine: more balanced, e.g., diltiazem
Adverse effects:
Myocardial depression
Hypotension
Inc HR with nifedipine; dec HR with verapamil, diltiazem
Flushing, ankle edema, headache
Therapeutic indications:
Angina: diltiazem, verapamil
Hypertension: nifedipine, diltiazem
Supraventricular tachycardia: verapamil
What are the 3 types of heart failure?
Left ventricular failure
Right ventricular failure
biventricular failure
What are the manifestations of HF?
Exertional dyspnea, fatigue, cough
Dependent edema
Enlarged heart
What are the drugs used to treat HF?
Diuretics
Inhibitors of RAAS: ACEI, ARB, aldosterone antagonists
Beta blockers
Vasodilators
Positive inotropic agents: cardiac glycosides (digitalis)
Where are cardiac glycosides sourced?
From plants
What is the chemical structure of a cardiac glycoside?
A molecule in which a sugar is bound to a non-carbohydrate (aglycone steroid nucleus) via a glycosidic bond.
Sugar: determines solubility
Aglycone steroid nucleus/non-carb: determines pharmacological activity
What is the mechanism of action of cardiac glycosides?
Digitalis directly inhibits Na+-K+-ATPase pump activity in cardiomyocytes
This increases intracellular Na+
This reverses the Na-Ca exchanger
Instead of pumping Ca out, the reversed exchanger brings more Ca into cell
This increases intracellular Ca
This enhances myocardial contractility (positive inotropy)
This increases vagal effect on the heart:
Decreased SA node firing rate (negative chronotropy)
Decreased conduction at AV node (negative dromotropy)
This decreases heart rate
What are the effects of digitalis on myocardial contractility and heart rate?
Increased myocardial contractility:
Dec heart size
Dec venous congestion
Dec edema
Inc renal perfusion
Decreased heart rate
What are the pharmacokinetics of digoxin?
Absorption: 70%
Onset: 1-2h
Peak: 6-8h
Half life: 40h
Duration: 3 days
Metabolism: liver (14% metabolized in liver)
Elimination: kidney (mostly excreted unchanged through kidney)
Digoxin accumulates in the body due to long duration and low amount metabolized
What are the therapeutic indications of digitalis/digoxin?
Congestive heart failure
Tachyarrhythmia: especially atrial flutter or atrial fibrillation
What are the drug interactions digoxin has?
K+:
Hyperkalemia decreases digitalis activity bc it competes for Na-K-ATPase
Hypokalemia increases digitalis activity
K losing diuretics increase digitalis activity and thus pose risk for toxicity
Hypercalcemia: inc risk of toxicity and dysrhythmia
Quinidine & Verapamil: decreased renal clearance & volume of distribution of digitalis increases digitalis level and toxicity
Antibiotics: inhibit intestinal flora that inactivate digoxin, increasing digoxin level.
What are the adverse effects of digoxin?
GI: anorexia, nausea, vomiting, diarrhea
CVS: sinus bradycardia, AV block, ventricular dysrhythmia
CNS: headache, fatigue, mental depression, confusion, hallucination, convulsions
Visual disturbances
Gynecomastia
What is the therapeutic range and toxic level for digoxin?
Therapeutic range: 0.8-1.6 ng/ml
Toxic level: >2.4 ng/ml
How is digoxin administered?
Route: oral or IV
Dose:
Rapid:
Loading dose: 1-1.5 mg over first day
Maintenance dose: 0.125-0.5 mg/day
Slow:
Start with maintenance dose of 0.125-0.5 mg/day
How is HF managed?
ABCD:
A: ACEI, ARB, Aldosterone antagonists, ARNI (angiontensin receptor-neprilysin inhibitor)
B: bed rest, beta blockers, natriuretic peptide
C: cardiac transplantation
D: diet, digitalis, diuretics, dilators
What does quadruple medical therapy of HF involve?
A combination of:
Angiontensin receptor-neprilysin inhibitors
Beta blockers
Aldosterone antagonists
Sodium/glucose cotransporter 2 inhibitor
Define hemostasis and thrombosis.
Hemostasis: the process that retains blood within vascular system.
Thrombosis: formation of a thrombus (blood clot) within a blood vessel
What are the steps of hemostasis:
Injury of a blood vessel causing loss of vascular integrity resulting in a series of 4 events:
Vasoconstriction
Platelet activation
Blood coagulation & clot formation
Fibrinolysis
Describe the role of vasoconstriction in hemostasis.
Dec blood flow to injured vessel = dec blood loss
Describe the role of platelet activation in hemostasis.
Platelets adhere to collagen fibrils on injured vessel
Platelets aggregate to each other under the effect of thrombin and fibrinogen, creating a platelet plug
Once activated, platelets release chemical mediators as ADP and thromboxane A2 to activate more platelets
Describe the role of blood coagulation and clot formation in hemostasis.
Activation of coagulation factors → coagulation pathway
Platelet plug turns into more stable blood clot (fibrin network + blood cells)
Describe the role of fibrinolysis in hemostasis.
The enzymatic process that dissolves the fibrin clot
Occurs simultaneously with coagulation
Controls the size and spread of fibrin clot
Carried out by the enzyme plasmin.
What are the 3 causes of bleeding disorders?
Vascular defects: e.g., vasculitis
Platelet defects: thrombocytopenia
Coagulation factor defects: hemophilias
Describe hemophilias.
Bleeding disorder related to a coagulation factor deficiency.
What are the 3 antithrombotic agents?
Antiplatelets: inhibit platelet function
Anticoagulants: inhibit coagulation factors
Thrombolytic agents: breakdown fibrin
List and describe the different kinds of antiplatelets.
Cyclooxygenase inhibitors:
Decrease cyclooxygenase enzyme, decreasing thromboxane A2
These include asprin and NSAIDS like ibuprofen
ADP receptor blockers:
Block ADP receptor, decreasing ADP-induced platelet aggregation
Safe when aspirin is contraindicated.
E.g., thienopyridines
Phosphodiesterase inhibitors:
e.g., Cilostazol
Selective decrease in PDE3, inc cAMP in platelets, increasing active protein kinase A, dec platelet aggregation
Used for intermittent claudication in peripheral vascular disease
Avoid in HF
Prostacyclin analogues:
inc activity of prostacyclin receptors, inc adenylyl cyclase enzyme, inc cAMP level in platelets
Inhaled or IV
E.g., iloprost, carbacyclin
Adenosine reuptake inhibitors:
inhibit adenosine reuptake by RBCs, platelets, and endothelial cells
Inc extracellular adenosine concentration, acting on receptors
Inc platelet cAMP synthesis
cAMP inhibits platelet aggregation
e.g., dipyridamole
Glycoprotein IIb/IIIa receptor blockers
Block glycoprotein IIb/IIIa receptors on platelets, dec platelet aggregation
Adverse: thrombocytopenia
Route: IV
e.g., a
What are the types of anticoagulants?
Heparin:
Unfractionated heparin
Low molecular weight heparin
Oral anticoagulants:
Warfarin & Dicoumarol
Direct thrombin inhibitors
Direct FXa inhibitors
Describe unfractionated heparin.
Augments the effect of antithrombin, inhibiting thrombin and factor Xa
Side effects:
Bleeding
Thrombocytopenia
Allergy
Osteoporsis
Route: IV or SC injection
Describe low molecular weight heparin.
More selective inhibition of factor Xa while sparing thrombin
Imrpoved pharmacokinetics
Less side effects
No need for coagulation monitoring
Administered SC
E.g., tinzaparin, enoxaparin
Describe warfarin.
Vitamin K is required for activation of many factors in the liver by carboxylation
To be effective, vitamin K has to be in reduced form under the effect of vitamin K epoxide reductase enzyme
Vitamin K epoxide reductase enzyme is inhibited by warfarin
Describe the differences in plasminogen binding, potential allergic reactions, antigenicity, risk of bleeding, plasma clearance (mins), and relative cost of streptokinase (a thrombolytic agent) and tissue plasminogen activator (t-PA)
Plasminogen binding:
Streptokinase = indirect
Tissue plasminogen activator = direct
Potential allergic reaction:
Streptokinase = yes
Tissue plasminogen activator = no
Antigenicity:
Streptokinase = high
Tissue plasminogen activator = low
Risk of bleeding:
Streptokinase = more
Tissue plasminogen activator = less
Plasma clearance:
Streptokinase: 15-25 mins
Tissue plasminogen activator = 4-8 mins
Relative cost:
Streptokinase = +
Tissue plasminogen activator = +++
Describe bronchial asthma.
Inflammation of bronchial walls, causing narrowing of airways and increased resistance to airflow.
What are 3 factors the increase airway obstruction?
Bronchoconstriction: contraction of bronchial smooth muscles
Mucosal edema: resulting from inflammation
Bronchiolar secretions: inc mucus secretion due to inflammation
What are the 2 types of anti-asthma agents?
Bronchodilators and anti-inflammatories.
What are the 3 classes of bronchodilators?
B2 adrenoreceptor agonists:
inc B2 adrenergic receptors on bronchial smooth muscles, resulting in bronchodilation
Dec mediators released from mast cells
Most effective bronchodilator
Short acting: salbutamol (albuterol) and terbutaline
Long acting: salmeterol and formoterol
Anticholinergics:
dec muscarinic receptors, blocking cholinergically-mediated bronchoconstriction
Usually adjunct therapy
Methylxanthines:
3 pharmacologically active compounds:
Caffeine
Theobromine
Theophylline:
Effect anti-asthma drug
dec phosphodiesterases, dec cAMP hydrolysis, resulting in accumulation of cAMP, relaxing bronchial smooth muscles, causing bronchodilation
Narrow therapeutic range with lots of CVS, CNS, and GI side effects
What are the 5 types of anti-inflammatory agents?
Glucocorticoids (steroids)
Mast cell stabilizers
Leukotriene inhibitors
Omalizumab
Methotrexate
Describe glucocorticoids
Anti-inflammatory effect on bronchial mucosa
dec macrophages, eosinophils, lymphocytes
dec mucus secretion
Mostly inhaled, also oral and IV
e.g., beclomethasone
Describe mast cell stabilizers.
Stabilize mast cells, dec release of chemical mediators (histamines) that cause bronchoconstriction, dec incidence of attacks
Used for prophylaxis, not suitable during acute attacks
Inhaled
Describe Leukotriene receptor antagonists.
Selective blocking of leukotrienes action on respiratory tract, resulting in dec mucus secretion and dec bronchoconstriction
Used as adjunct therapy and for prophylaxis (not suitable during acute attacks)
Orally administered
Describe leukotriene synthesis inhibitors.
dec 5-lipoxygenase enzyme, dec leukotrienes production
Used as adjunct therapy and for prophylaxis
Not good for liver
Administered orally
Describe omalizumab.
Recombinant anti-IgE antibody
Binds IgE, inhibiting the binding of IgE to the high-affinity IgE receptor
Used in severe, persistent allergic asthma uncontrollable with steroids
Administered via SC injection every 2-4 weeks.
Can cause anaphylaxis.
Describe methotrexate.
A cytotoxic drug mainly used for treatment of malignancies and autoimmune diseases
Significant side effects, so limit its use (liver toxicity, bone marrow failure)
Oral or parenteral injection