aliphatic hydrocarbons
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Whats a hydrocarbon? Whats it divided into?
organic compounds of H and C only
Divided into aliphatic (non aromatic ) and aromatic type
Aliphatic - straight, branches or non aromatic cyclic structures
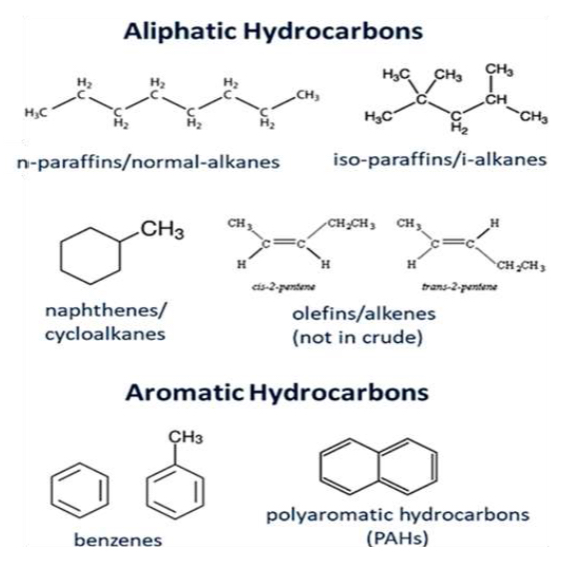
Define alkane, alkene, alkynes
Alkane - saturated (single bond C-C ), inert
Alkene - unsaturated, reactive one or more double bonds (c=c)
Alkyne - unsaturated, highly reactive one or more triple bonds (C=-C)
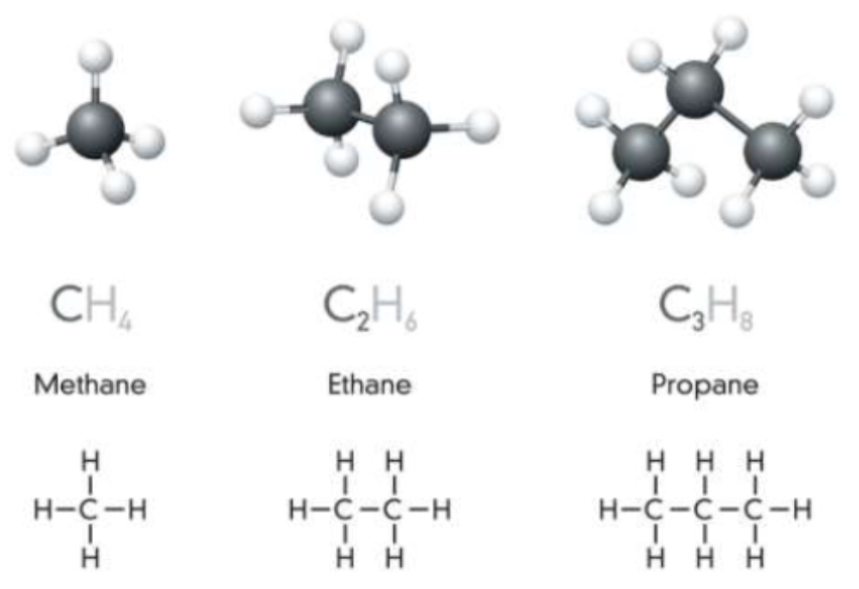
Whats the general formula of alkane and their structure and nomenclature? Draw methane, ethane, propane
Linear, branched or cyclic
IUPAC prefix - carbon number and ANE
general formula CnH2n+2
Physical properties of alkane
non polar and insoluble in water
Boiling point increases w/ molecular size
Common as fuels and solvents
Chemical properties of alkanes
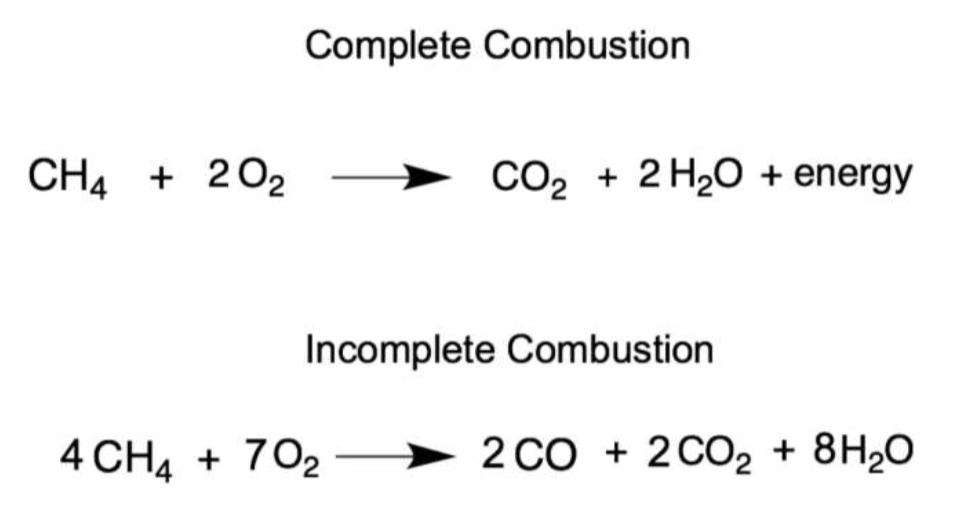
combustion - burn in oxygen
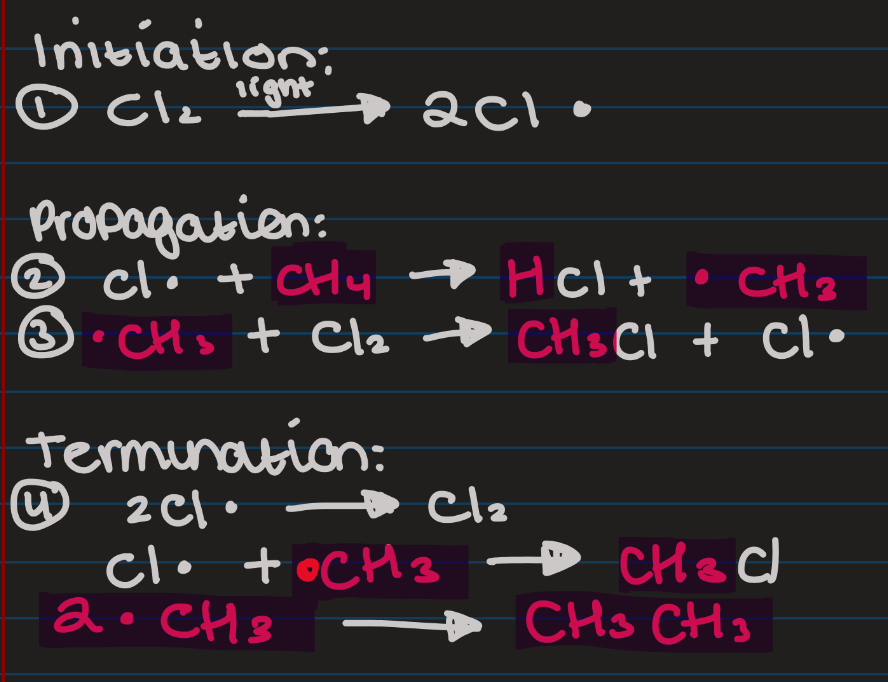
Halogenation - halogen added
Low reactivity
Show complete combustion and incomplete combustion
Show Halogenation (free radical substitution) of chlorine and CH4
Describe low reactivity of alkanes.
alkanes due to their chemical inertness, hydrophobic nature and low reactivity and occasionally used as inert carriers of excipients in pharmaceutical formulations
Low reactivity - how do alkane solved or carriers work in drug delivery? Eg.
Alkanes such as liquid paraffin (mixture of long chain alkanes) used in:
oral laxatives
Topical creams/ ointments (as emollients)
Suspension vehicles for lipophilic drugs
Petroleum jelly is semisolid mixture of alkanes from c16 to c20 and are used as a base in:
skin protectants
Antibacterial ointments
Cosmetic formulations
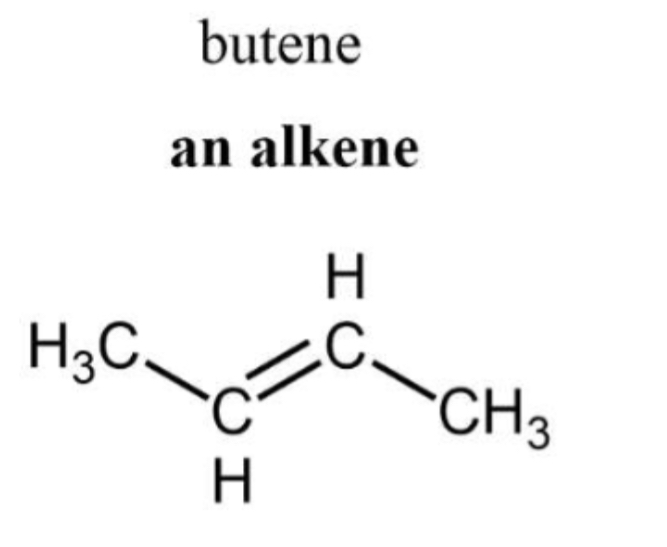
General formula and structure and nomenclature of alkenes And draw butene
Contain at least a C-C double bond
ENE suffix and indicate position of double bond
general formula CnH2n
Physical properties of alkenes
similar to alkanes - but slightly more polar
Boiling/melting points increase w/ size
Double bonds affect electron distribution
Chemical properties of alkenes
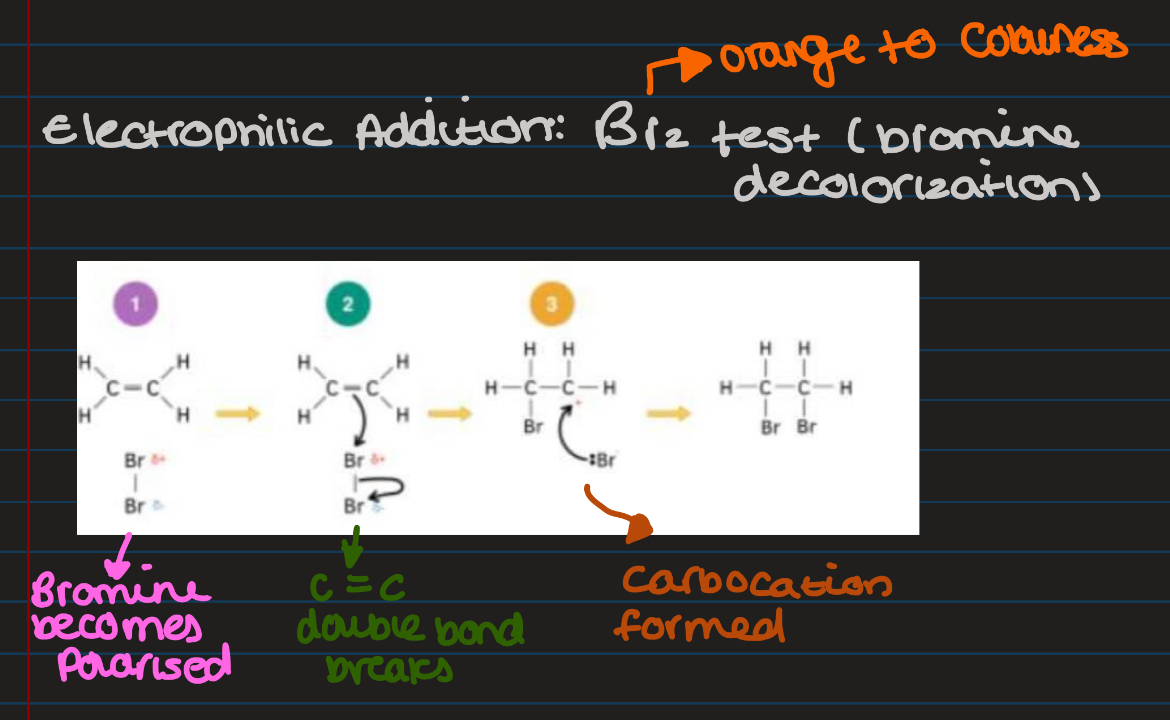
electrophilic addition
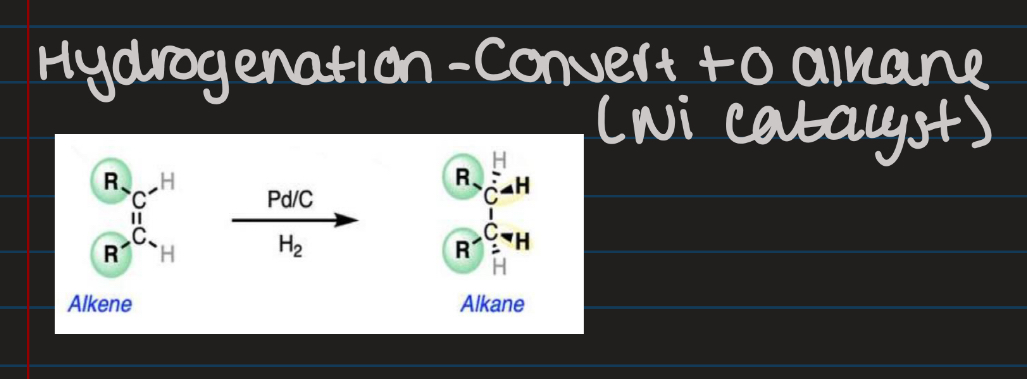
Hydrogenation
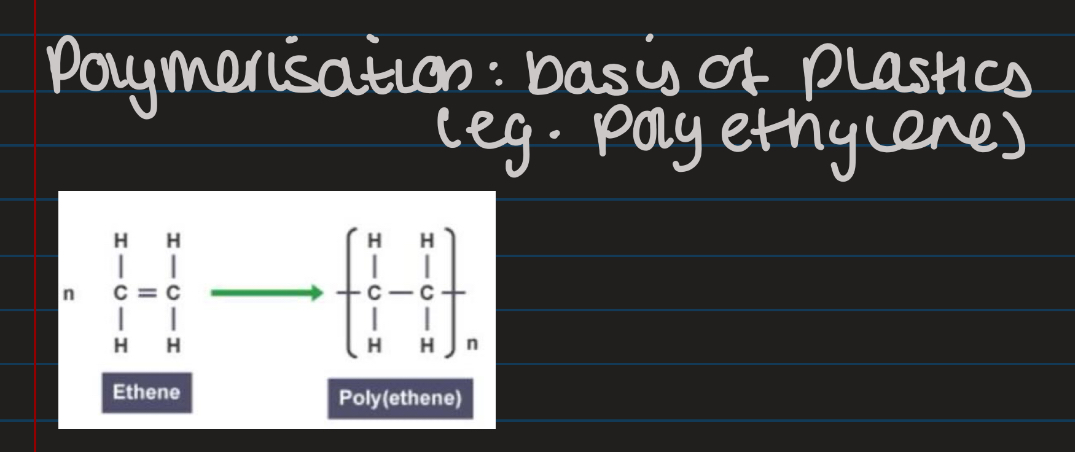
Polymerisation
Do electrophilic addition with ethene
Do hydrogenation of ethene
Do polymerisation of ethene
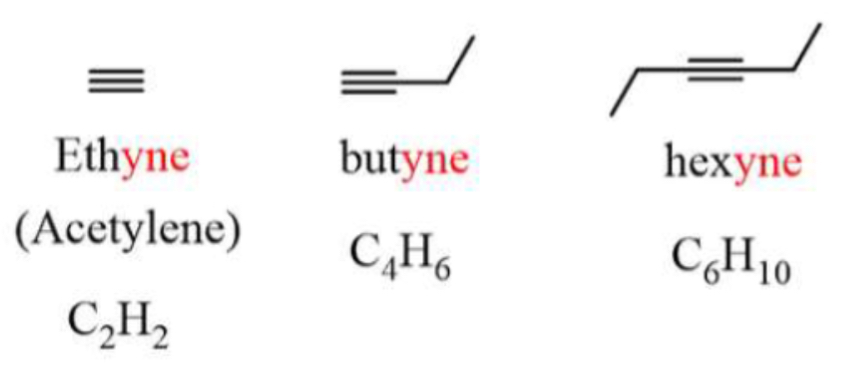
Genera formula and structure and nomenclature of alkynes. Draw ethene and butyne and hexyne
triple bond - linear geometry
Named with YNE and specify bond location
General formula CnH2n-2
What reactions and properties does alkynes have?
addition reactions similar to alkenes
Precursor for complex pharmaceuticals
Do addition reactions for propyne
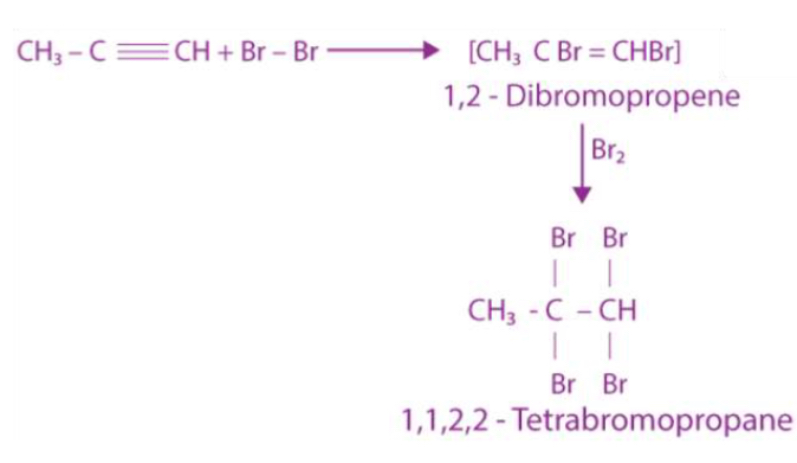
How do alkynes act as precursors in complex pharmaceuticals?
Alkynes serve as versatile scaffold that can be transformed into variety of functional groups:
hydrogenation —> alkenes or alkanes
Hydrohalogenation —> vinyl halides
Hydration —> ketones or aldehydes
Cycloaddition —> aromatic or heterocyclic systems
These transformations allow alkynes to be foundational in stepwise synthesis of complex pharmaceuticals
How to hydrocarbons have relevance to pharmacy?
hydrocarbon chains used in local anaesthetic like lidocaine
Used in ointment, bases , creams and gels
Drug delivery - enhances membrane permeability
What are the safety and environmental concerns for hydrocarbons?
volatility —> inhalation risks
Highly flammable - strict handling needed
Environmental concerns —> non biodegradability, pollution
Which is an alkene? Ethane, ethene, ethyne
Alkanes are saturated or unsaturated?
Alkyne general formula?
Ethene
Saturated
CnH2n-2