Isomerism in Pharmaceutical Organic Chemistry
1/177
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
178 Terms
Isomerism is the phenomenon where more than one compound has the same chemical formula but different chemical structures.
What is isomerism?
Isomers are chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecules.
What are isomers?
The term 'isomer' is derived from the Greek words 'isos' meaning equal and 'meros' meaning parts.
What does the term 'isomer' derive from?
Skeletal isomerism, also known as chain isomerism, involves different carbon chain structures where isomers differ in the branching of carbon.
What is skeletal isomerism?
The different arrangement of isomers can lead to very different chemical and physical properties.
What is the importance of isomerism in organic chemistry?
The two primary types of isomerism are structural isomerism and stereoisomerism.
What are the two primary types of isomerism?
Structural isomerism, or constitutional isomerism, refers to isomers that have different arrangements of atoms and functional groups.
What is structural isomerism?
The four subtypes of structural isomerism are chain isomerism, position isomerism, functional group isomerism, and tautomeric isomerism.
What are the four subtypes of structural isomerism?
Functional isomerism refers to compounds that have the same chemical formula but different functional groups attached.
What is functional isomerism?
Stereoisomerism arises in compounds that have the same chemical formula but different orientations of atoms in three-dimensional space.
What is stereoisomerism?
Stereoisomers are compounds that exhibit stereoisomerism.
What are stereoisomers?
An example is a straight chain alkane and a cycloalkane, both having the same molecular formula but different structures.
Give an example of a compound that can exhibit functional isomerism.
Jacob Berzelius coined the term 'organic' in the year 1830.
What is the significance of Jacob Berzelius in organic chemistry?
The molecular formula for pentane is C5H12.
What is the molecular formula for pentane?
An example is an alcohol and an aldehyde, both with the formula C6H6 but different structures.
What is an example of a structural isomer with the same molecular formula but different functional groups?
As the number of carbon atoms in a molecule increases, the number of possible isomers also increases.
What is the relationship between the number of carbon atoms and the number of possible isomers?
All isomers have the same molecular formula but differ in the structural or spatial arrangement of atoms.
What is a common characteristic of all isomers?
Functional isomerism involves different functional groups.
What type of isomerism involves different functional groups?
Constitutional isomerism refers to structural isomerism where the functional groups and atoms are linked in different ways.
What does the term 'constitutional isomerism' refer to?
In chain isomerism, branching refers to the different arrangements of carbon chains, leading to distinct isomers.
What is the role of branching in chain isomerism?
In stereoisomerism, the arrangement of atoms in three-dimensional space results in different spatial configurations.
What is the significance of the arrangement of atoms in stereoisomerism?
Different structural arrangements can lead to variations in chemical and physical properties.
What can be a consequence of different structural arrangements in isomers?
The position of a functional group differs on the same carbon chain.
What is position isomerism?
Isomers that differ in the arrangement of atoms or groups within the same molecular formula.
What are structural isomers?
Isomers that differ in the arrangement of the carbon skeleton.
What are chain isomers?
A type of isomerism where two or more compounds called tautomers can easily convert into each other by a simple chemical reaction, usually involving the shift of hydrogen atoms and a double bond.
What is tautomerism?
Tautomers are typically in equilibrium, meaning they constantly change back and forth under normal conditions.
What is a key feature of tautomers?
Proton transfers occur in an intramolecular fashion, leading to isomers that differ only in the positions of protons and electrons.
What is the significance of proton transfer in tautomerism?
It involves the transfer of protons and double bonds.
What does tautomerism involve?
Keto-enol tautomerism, where a ketone can be converted into an enol.
What is the most common type of tautomerism?
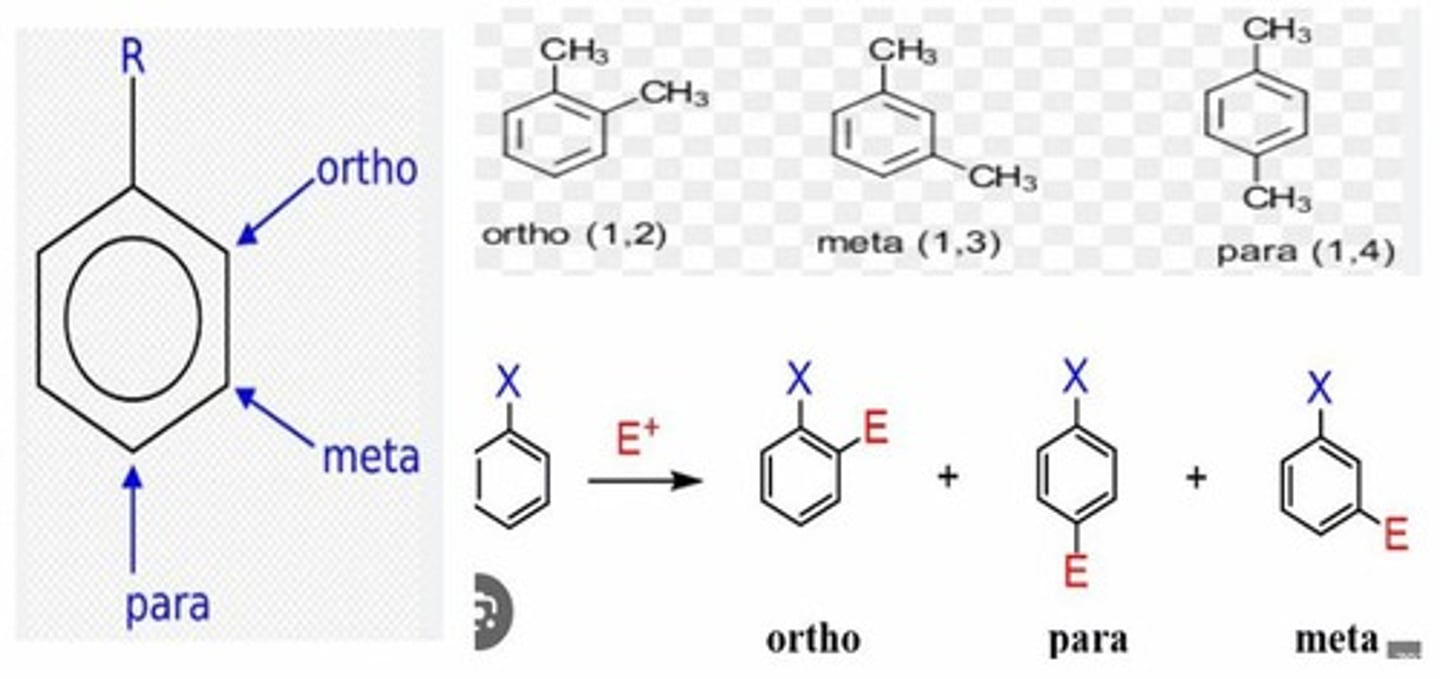
They refer to the relative positions of substituents on a benzene ring: ortho (adjacent), meta (separated by one carbon), and para (opposite sides).
What are ortho, meta, and para in the context of benzene rings?
Isomers that have the same molecular formula and structure but differ in the arrangement of atoms or groups in three-dimensional space.
What are stereoisomers?
Conformational isomers and configurational isomers.
What are the two types of stereoisomers?
Different spatial arrangements of atoms in a molecule resulting from rotations around single bonds, without breaking any bonds.
What are conformational isomers?
Steric hindrance and torsional strain.
What influences the rotation in conformational isomers?
Newman's projection and sawhorse projection.
What are the two key representations used to visualize conformations?
C6H6.
What is the chemical formula of benzene?
The relative position of non-hydrogen substituents determines the type of positional isomers.
How do substituents affect positional isomers in benzene?
Ortho substituents are adjacent (positions 1 and 2), while para substituents are on opposite sides of the ring (positions 1 and 4).
What is the difference between ortho and para substituents?
Functional groups determine the reactivity and can change the isomeric form based on their position.
What is the role of functional groups in isomerism?
The process gives more stability to the compound and is reversible.
What happens during the tautomerism process?
It usually involves the shift of hydrogen atoms and a double bond.
What is a characteristic of the chemical reaction involved in tautomerism?
The locant indicates the position of the functional group attachment in the molecule.
What is the significance of the locant in isomer names?
Conformers differ by rotation around single bonds, while configurational isomers require bond breaking to interconvert.
What is the difference between conformers and configurational isomers?
The position can affect the stability and reactivity of the molecule.
What is the impact of the position of functional groups on molecular stability?
A specific spatial arrangement of atoms in an open chain molecule that can be interconverted by rotation around single bonds without breaking any chemical bonds.
What are acyclic conformers/compounds?
Isomers that rapidly interconvert, often with a hydrogen atom shifting between atoms, differing only in the position of protons and electrons.
What are tautomers?
They exist together in equilibrium and easily interchange via an intramolecular proton transfer.
How do tautomers typically exist?
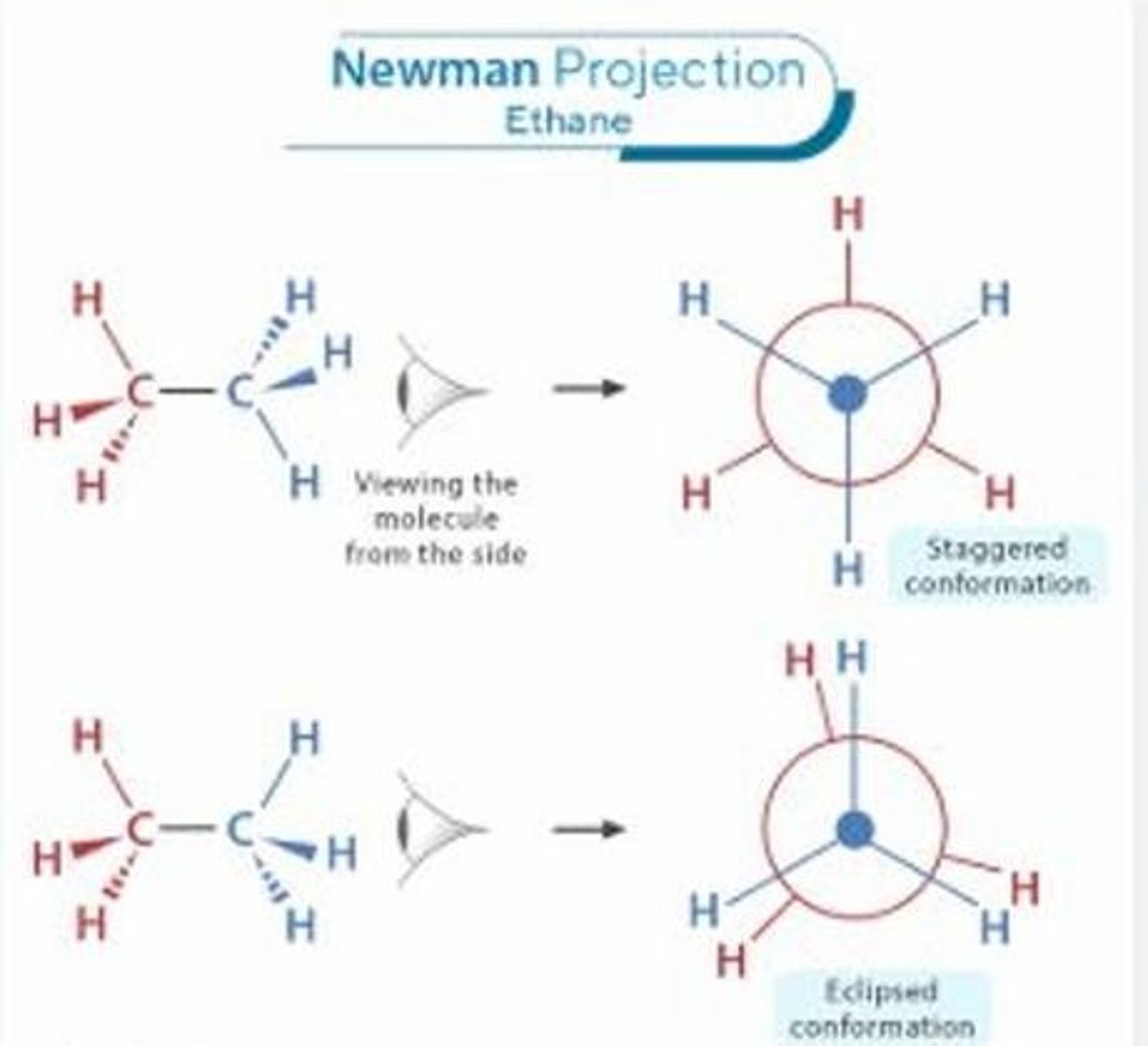
To visualize the spatial arrangement of atoms around a carbon-carbon bond by looking directly down the bond.
What is the purpose of Newman's Projection Formula?
The specific three-dimensional arrangement of atoms within a cyclic ring molecule while maintaining the same bond connectivity and configuration.
What is the definition of cyclic conformers?
Chair Conformation, Twist-Boat Conformation, Boat Conformation.
Name three types of cyclic conformers.
Wedge and Dash Projection.
What is the Flying Wedge Projection also known as?
A bond above the plane of the paper.
What does a solid wedge represent in Flying Wedge Projection?
A bond below the plane of the paper.
What does a dashed wedge represent in Flying Wedge Projection?
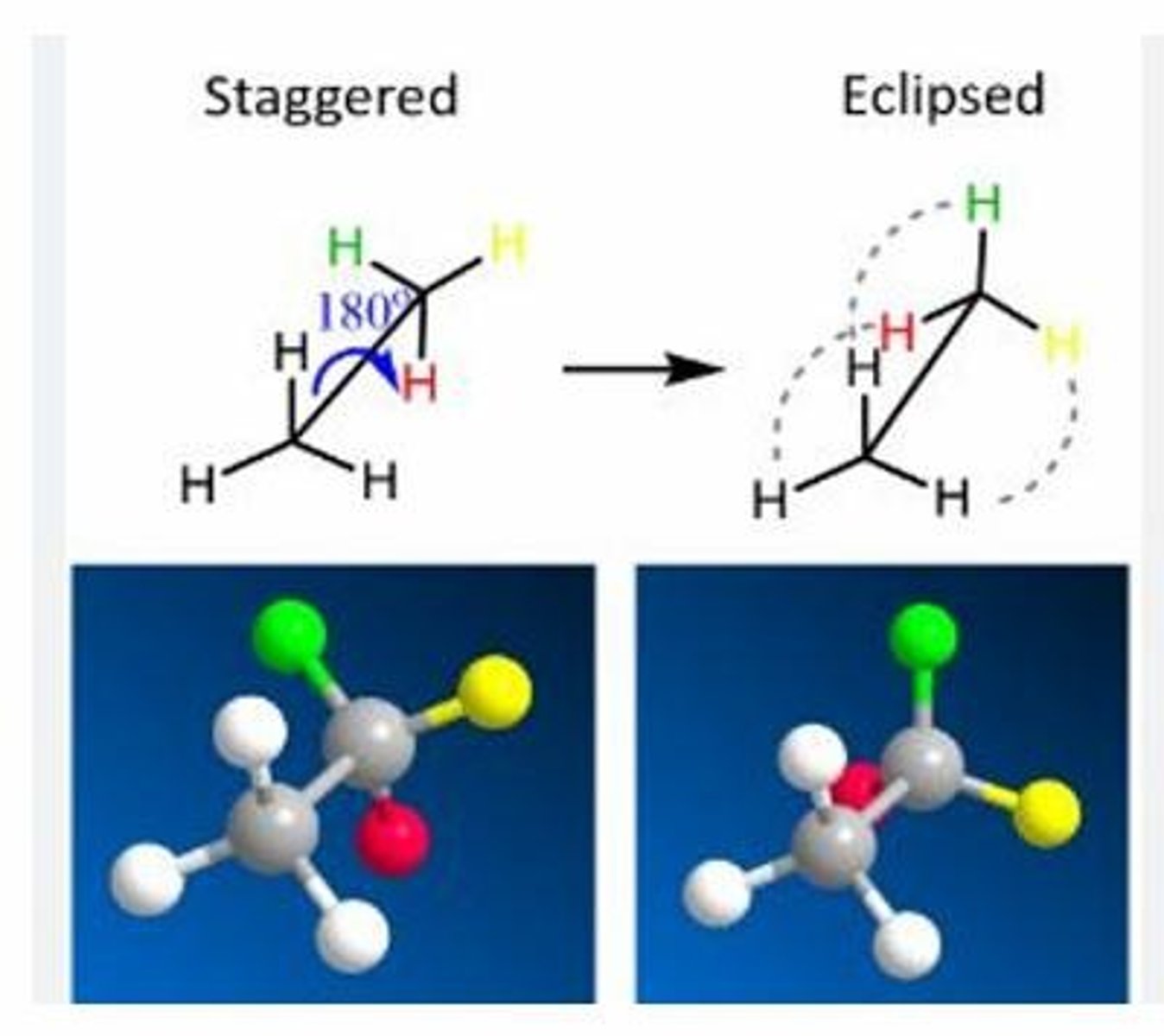
Atoms or groups attached to the carbons are as far apart as possible, minimizing steric strain and resulting in a more stable conformation.
What is the significance of staggered conformation in Newman Projection?
60 degrees.
What is the dihedral angle in staggered conformation?
A conformation where atoms or groups attached to the carbons are aligned with each other, causing torsional strain and making it less stable.
What is eclipsed conformation in Newman Projection?
0 degrees.
What is the dihedral angle in eclipsed conformation?
Different spatial arrangements of the same molecule that can be interconverted by rotation around a single bond.
What are conformational isomers?
A dot in the center.
What is the representation of the front carbon in Newman's projection?
The carbon behind the front carbon.
What does the larger circle represent in Newman's projection?
The bonds and substituents or atoms/group of atoms attached to each carbon.
What do the lines extending from the front and back circles represent in Newman's projection?
To examine the relative positions of the atoms or groups attached to each carbon.
What is the purpose of using Newman's projection?
It is more stable and lower in energy due to minimized repulsive interactions.
What happens to energy levels in staggered conformation?
It has higher energy due to increased strain from substituents directly overlapping.
What happens to energy levels in eclipsed conformation?
Minimizing steric hindrance leads to lower energy and more stable conformations.
What is the role of steric hindrance in molecular stability?
Molecules containing a chiral center.
What type of molecules is the Flying Wedge Projection typically used for?
Higher energy due to increased strain when substituents directly overlap.
What is the key feature of the Newman projection regarding energy?
A dot in the center.
What does the front carbon represent in a molecular projection?
A circle around the dot.
How is the back carbon represented in a molecular projection?
To visualize staggered and eclipsed conformations of molecules, which are crucial for understanding their stability.
What is the purpose of using molecular projections in chemistry?
The spatial arrangement of substituents on adjacent carbons, including staggered and eclipsed conformations.
What does the Sawhorse projection help visualize?
By a diagonal line (or horizontal line).
How is the front carbon represented in a Sawhorse projection?
By a second diagonal line.
How is the back carbon represented in a Sawhorse projection?
From an oblique angle similar to a side view.
What is the viewing perspective of the Sawhorse projection?
Approximately 60 degrees.
What angle do the groups attached to the carbons in a Sawhorse projection typically show?
Staggered and eclipsed conformations.
What are the two types of conformations visualized in the Sawhorse projection?
Staggered has hydrogen atoms positioned between those on the back carbon, creating a lower energy conformation; eclipsed has hydrogen atoms directly aligned, resulting in a higher energy conformation due to steric hindrance.
What is the difference between staggered and eclipsed conformations in a Sawhorse projection?
Stereoisomers that interconvert by breaking chemical bonds, differing in spatial arrangement around a specific bond or center.
What are configurational isomers?
Conformational isomers arise from rotation around single bonds and can interconvert easily, while configurational isomers require breaking and reforming bonds to interconvert.
What is the key difference between conformational and configurational isomers?
Enantiomers.
What are optical isomers also known as?
They differ in their optical activities.
What distinguishes enantiomers from each other?
It offers a 3D perspective of the molecule, making it easier to visualize staggered and eclipsed conformations.
What is a key feature of the Sawhorse projection?
To show the relative positions of substituents on each carbon.
How can the angle between bonds in the Sawhorse projection be adjusted?
It makes it easier to visualize complex bond arrangements.
Why is the Sawhorse projection useful for larger molecules?
Cyclic molecules, such as cyclohexane.
In what type of molecules is the Sawhorse projection helpful for understanding axial and equatorial positions?
They can exist as non-superimposable mirror images.
What is the significance of chiral centers in optical isomers?
Interconversion requires breaking and reforming covalent bonds.
What is the relationship between configurational isomers and covalent bonds?
Configurational isomers.
What type of isomers are C-strand isomers of alkanes?
Bonds are shown as diagonal lines radiating from the carbon lines.
What is the visual representation of bonds in a Sawhorse projection?
Dextro enantiomers rotate the plane of polarized light to the right, while laevo enantiomers rotate it to the left.
What is the difference between dextro and laevo enantiomers in terms of their effect on polarized light?
A chiral center is a carbon atom that has four different atoms or groups attached to it.
What is a chiral center?
Newman projection and Sawhorse projection.
What are the two methods used to visualize the spatial arrangement of atoms around a C-C bond?
Newman projection views the molecule directly down the C-C bond, while Sawhorse projection provides a different perspective that can enhance clarity.
How does the viewpoint differ between Newman and Sawhorse projections?
Optical activity refers to the ability of enantiomers to rotate plane-polarized light in opposite directions.
What is optical activity in the context of enantiomers?
Yes, enantiomers have the same physical properties, such as melting point and boiling point, but can interact differently with other chiral molecules.
Do enantiomers have the same physical properties?
L-alanine and D-alanine.
Give an example of a pair of enantiomers.
A racemic mixture contains equal amounts of both enantiomers and does not rotate plane-polarized light.
What is a racemic mixture?
Dextrorotatory (+) for clockwise rotation and levorotatory (-) for anticlockwise rotation.
What are the two designations used to indicate the rotation of plane-polarized light?
Non-superimposable mirror images indicate that a molecule is chiral, meaning it cannot perfectly align with its mirror image.
What is the significance of non-superimposable mirror images in chirality?