Topic 7 - Nucleophilic Substitution at Carbon Reactions
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
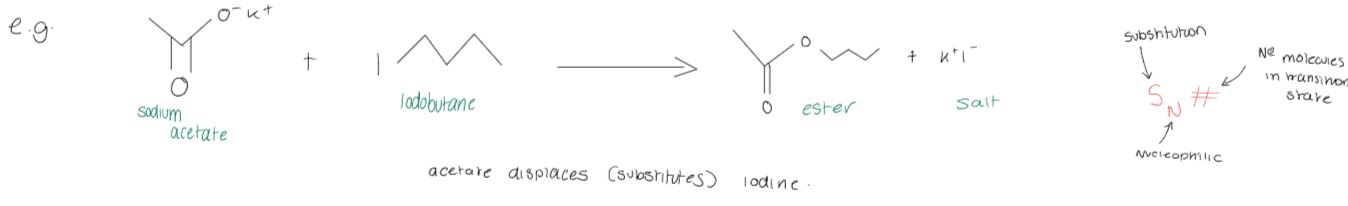
What is nucleophilic substitution?
Nucleophilic substitution is a chemical reaction where a nucleophile replaces a leaving group in a substrate molecule. It proceeds via two main mechanisms: SN1 and SN2.
What is the SN2 reaction profile?
The SN2 reaction profile describes a one-step mechanism where the nucleophile attacks the substrate and displaces the leaving group simultaneously.
The product(s) feature a new bond between the nucleophile and the electrophile. The leaving group bond breaks and leaves with 2 electrons.
The nucleophile donates 2 electrons to the empty antibonding sigma orbital of the electrophile.
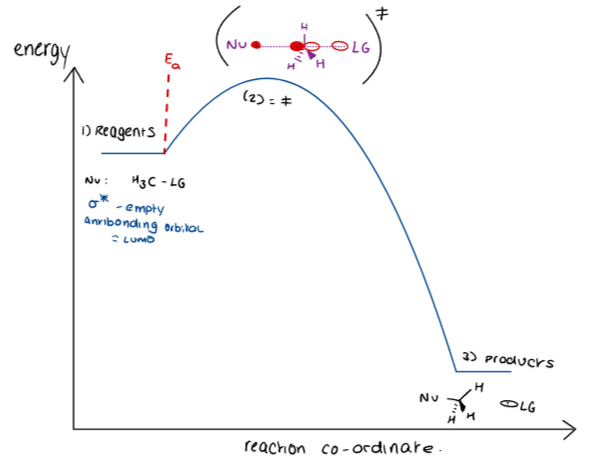
What is the transition state?
The transition state is a high-energy, unstable arrangement of atoms that occurs during the course of a chemical reaction, representing the point at which reactants are transformed into products.
It is represented by the symbol ‡.
There are partial bonds to the nucleophile and leaving group, with them both at a 180 degree bond angle.
The reacting centre is flattened (sp2 hybridisation).
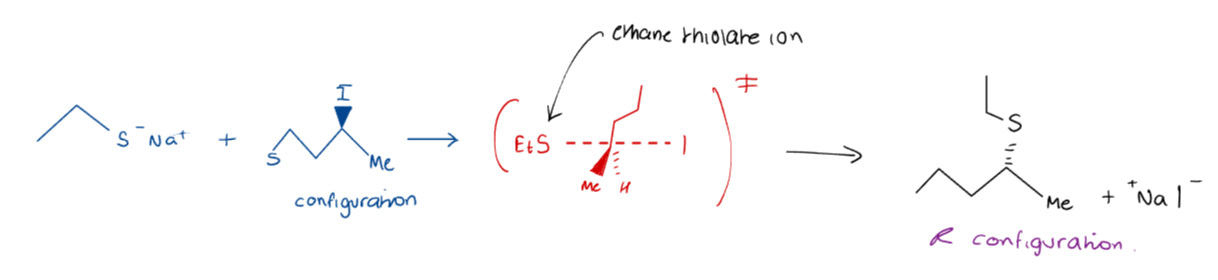
This leads to inversion of configuration (i.e. flipping of R and S).
How can nucleophile activity by increased in SN2 reactions?
Use an aprotic solvent as they do not form hydrogen bonds, which means that the nucleophile stays free and able to react.
Reduce steric hindrance at the electrophile, which makes nucleophilic attack easier (i.e. less branched electrophile means that it is easier for nucleophiles to react).
What occurs in a SN2 reaction?
In an SN2 reaction, a nucleophile attacks the electrophile, resulting in the displacement of the leaving group. This mechanism involves a single transition state.
Only when the electrophile is chiral is it possible to observe a SN2 reaction, as the stereochemistry is inverted, producing the opposite configuration.
How does the concentration of nucleophiles and electrophiles affect the rate of a SN2 reaction?
In SN2 reactions, increasing the concentration of either the nucleophile or electrophile raises the reaction rate.
They are both the rate determining state, acting as a bimolecular transition state.
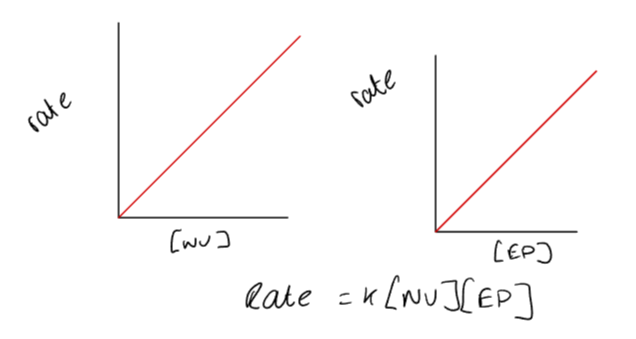
How does substitution affect the rate of SN2 reactions?
Substitution affects the rate of SN2 reactions by influencing the steric hindrance around the electrophile.
Larger functional groups attached to the C-LG bond slow down the overall SN2 rate.
-→ This is because they prevent the nucleophile approaching σ* and because of steric repulsion.
However, substitution at tertiary centres can occur.
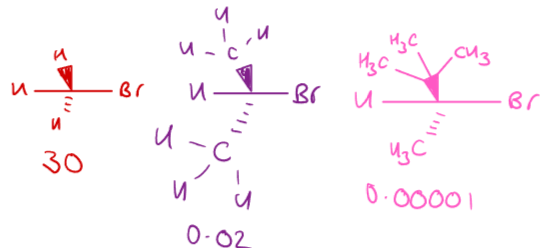
What are leaving groups?
Leaving groups are atoms or groups that detach from the substrate during a nucleophilic substitution reaction.
Halides are good examples of leaving groups.
Generally, the more stable the leaving group, the better.
→ Lower pKa values indicate leaving group ability.
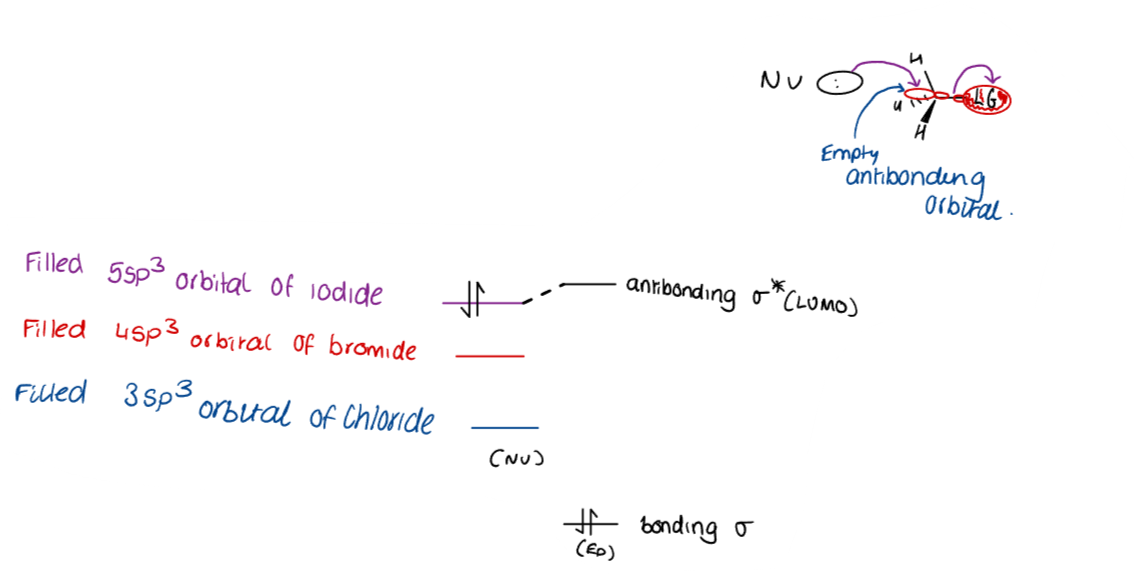
What makes a good nucleophile?
Higher pKa indicates better nucleophilicity.
→ The bulkier and more stable, the worse of a nucleophile.
What is meant by relative nucleophilicity?
Relative nucleophilicity refers to the comparison of the strength or ability of different nucleophiles to donate electron pairs in nucleophilic substitution reactions.
Reactions are fastest when the gap between HOMO and LUMO is the smallest.
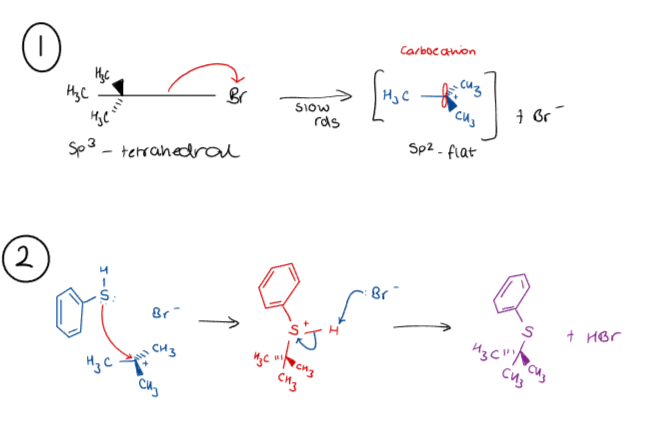
What is the mechanism for substitution at tertiary centres?
(1) With sufficient energy, an electron withdrawing group will undergo heterolytic fission.
Two electrons from the σ bond are transferred to the leaving group, which requires energy (hence the rate determining step).
(2) A nucleophile then donates two electrons to the carbocation to form a new bond.
As the intermediate is planar, the nucleophile can add to either side.
The stereochemistry is destroyed, and a new tetrahedral sp3 centre is formed in the product.
The new centre may be chiral, but will be racemic.
This is a SN1 reaction.
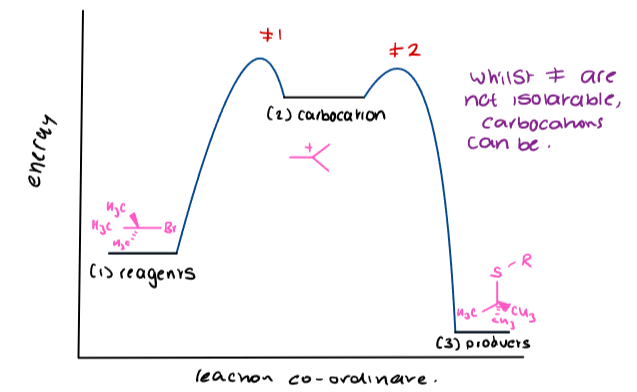
What is the SN1 reaction profile?
The SN1 reaction profile illustrates the two-step mechanism of nucleophilic substitution at tertiary centres, showing the formation of a carbocation intermediate and the subsequent reaction with a nucleophile.
The high activation energy barrier is primarily associated with the first step of carbocation formation, making it the rate-determining step.
How does the concentration of nucleophiles and electrophiles affect the rate of a SN1 reaction?
The rate varies linearly with respect to [Ep], and is unaffected by [Nu].
This is evidence that only the electrophile is involved in the rate determining step, a unimolecular transition state.
In SN1, the C-LG bond breaks, and 2 electrons are donated to the leaving group. This is the slow step, dependent on the [Ep].
![<p>The rate <strong>varies linearly with respect to [Ep]</strong>, and is <strong>unaffected by [Nu]</strong>.</p><ul><li><p>This is evidence that only the electrophile is involved in the rate determining step, a <strong>unimolecular transition state</strong>.</p></li></ul><p>In S<sub>N</sub>1, the C-LG bond breaks, and<strong> 2 electrons are donated to the leaving group</strong>. This is the slow step, dependent on the [Ep].</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/2205bed4-24a1-495f-bf17-78e3ea92e634.png)
What is the mechanism for a SN1 reaction?
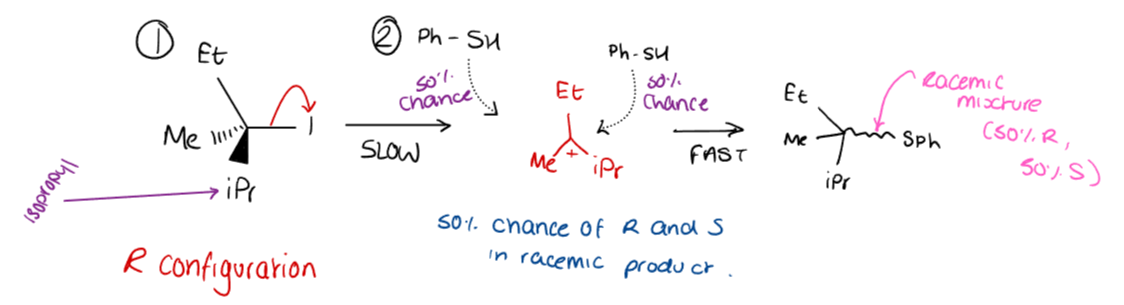
How does carbocation stability affect SN1 reactions?
The more substituted the electrophilic centre, the more stable the carbocation centre is, which favours the SN1 reaction.
The more substituted a carbocation, the more stabilised it is, and the more likely a SN1 reaction becomes, as the activation energy is lower.
→ Tertiary carbocations have many adjacent σ bonds, and the donation of electron density towards the carbocation stabilises it.
This mechanism is called hyperconjugation, i.e. orbital overlap between adjacent σ bonds and the empty p-orbital.
Less substituted carbocations (e.g. primary) are less stable. SN1 reactions not favourable as the Ea is higher.
→ There are no adjacent σ bonds available for hyperconjugation.
How does resonance stabilisation of a carbocation accelerate a SN1 reaction?
When a carbocation forms, it has a positive charge on a carbon atom. This positively charged carbon is very unstable and reactive, but resonance stabilisation can help by spreading out the positive charge over more atoms, increasing stability.
A more stable carbocation will form more readily, which means the rate-determining step will happen faster.
Since the carbocation is stabilised by resonance, the activation energy is lower. This means the reaction proceeds faster.