Aromatic Substitution Reactions
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
what is the mech for EAS
a benze ring acts as a nucleophile and attacks an elec, this forms a sigma complex where resonance structures move a pos chare around the ring from which a base can come and deprotonate the ring where the elec. is attached to form an elimination reaction and restoring aromaticity to the ring and the elec. replaces the H that was there.
what are reagents for chloronation and bromination
cl2 or br2 albr3, or alcl3
what are reatents for nitration and sulfonation
Hno3, h2so4, so3, h2so4
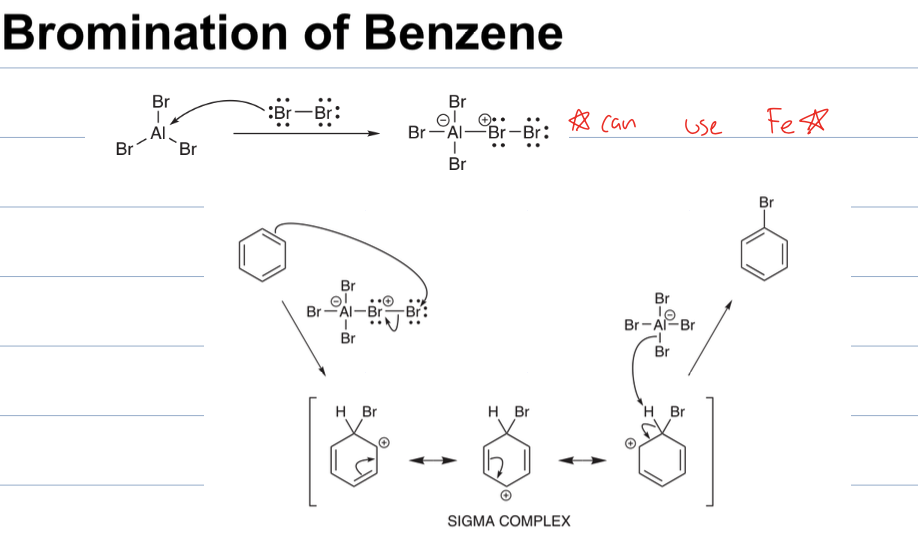
what is rxn with AlBr3 and Br2
EAS
where a Br is added to the benzene and Br2 attacks AlBr3 to become electrophilic.
The Br is attatched to the ring and AlBr4 is what deprotonates the ring to restore aromaticity.
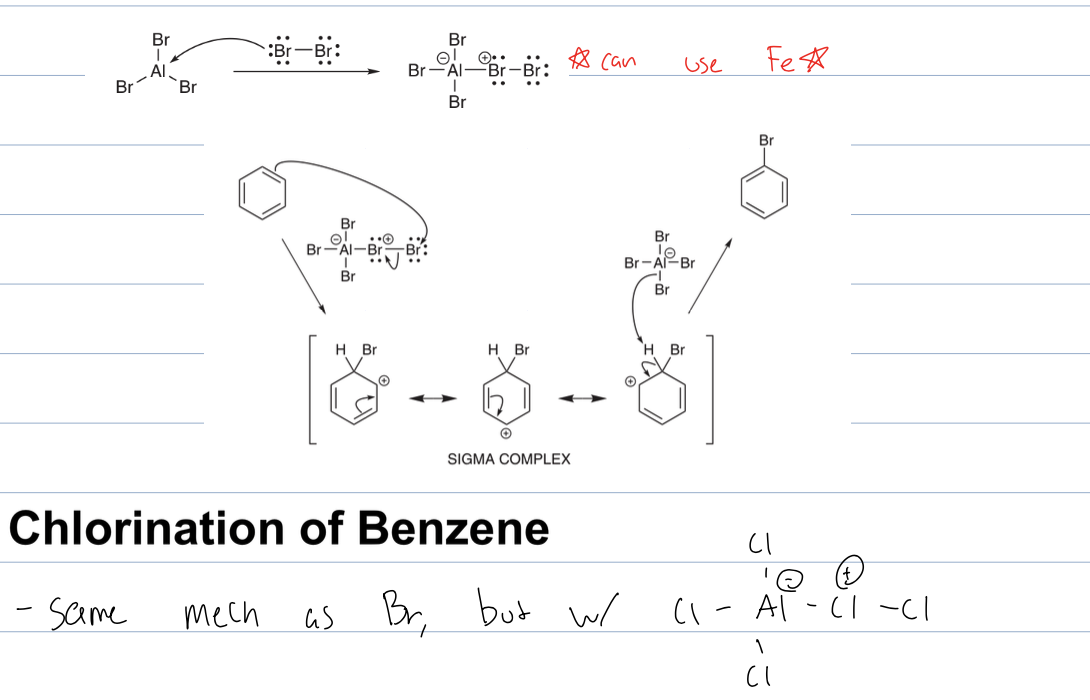
what is rxn with AlCL3 and Cl2
EAS
where a CL is added to the benzene and Cl2 attacks AlCl3 to become electrophilic.
The Cl is attatched to the ring and AlCl4 is what deprotonates the ring to restore aromaticity.
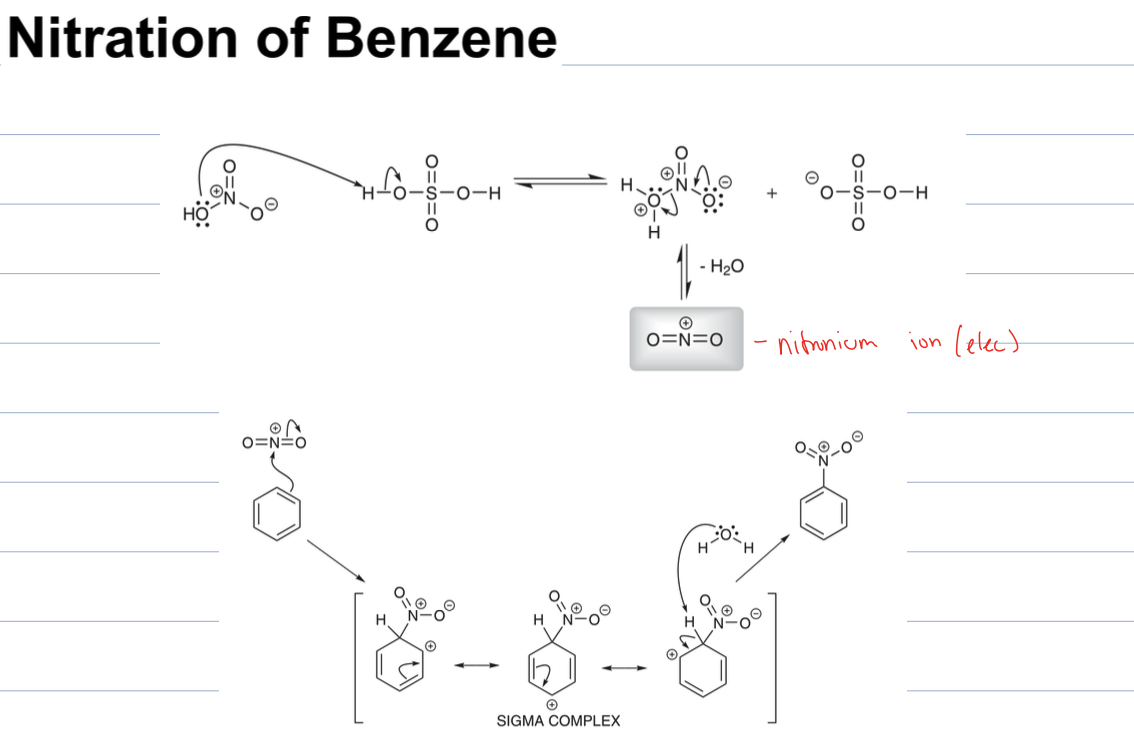
what is rxn with HNO3 and H2So4
nitration of benzene
where a NO2 group is added to benzene
the OH group of HNO3 deprotonates H2SO4 to form the nitronium ion (NO2+), which acts as the electrophile in the EAS reaction.
then the benzene attacks N and then forms sigma complex. the water left over deprotonates the sigma complex, restoring aromaticity and yielding nitrobenzene.
what rxn with fuming sulfuric acid (H2SO4 and SO3)
sulfonation of benzene where a HSO3 group is added to benzene. The benzene attacks the S to form the sigma complex with one of the O bearing a neg. charge. then water depronates the sigma complex to restore aromaticity to the ring. and then another proton transfer to get HSO3 (bc one O has neg. charge)
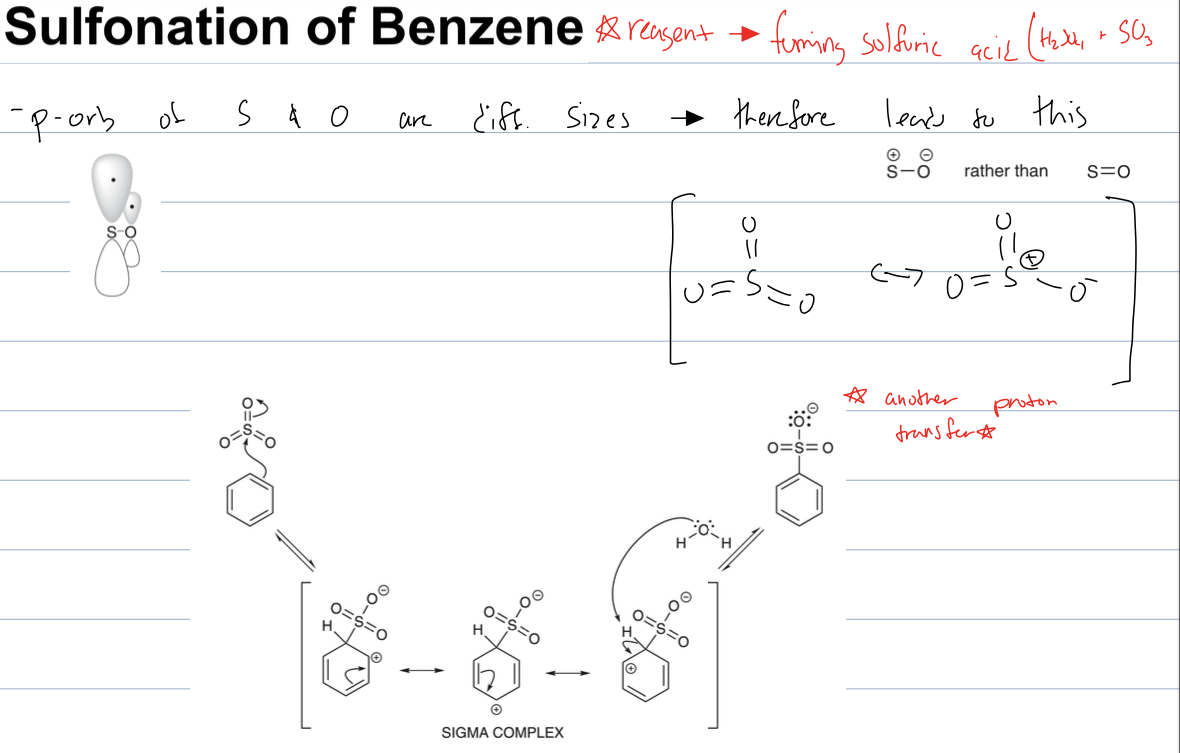
why does SO3 act as electrophile in sulfonation of benzene
the p orb of S and O are different sizes, therefore leads to S being pos charged and O being negtively charged rather than S being double bonded to O
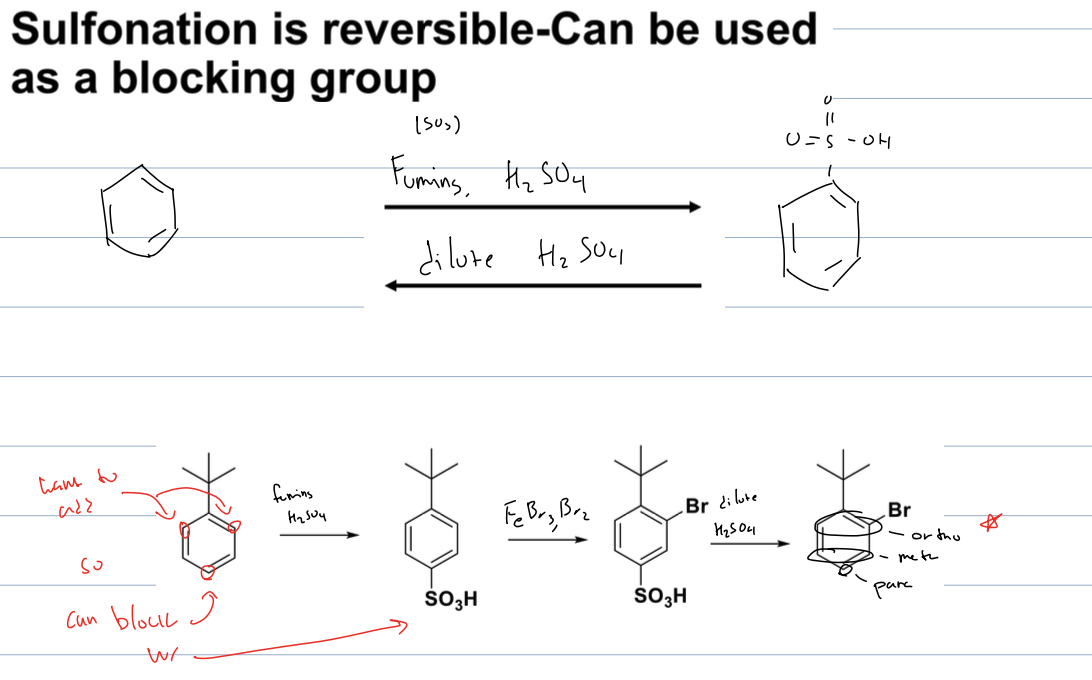
how can sulfonation be used as a blocking group for ortho poitions
if you use fuming H2SO4, the SO3H will be added at para position. Then can use any other EAS mech to add at ortho positions. then you can remove the SO3H group with dilute acid, restoring the aromatic structure. and having new addtion to ortho position
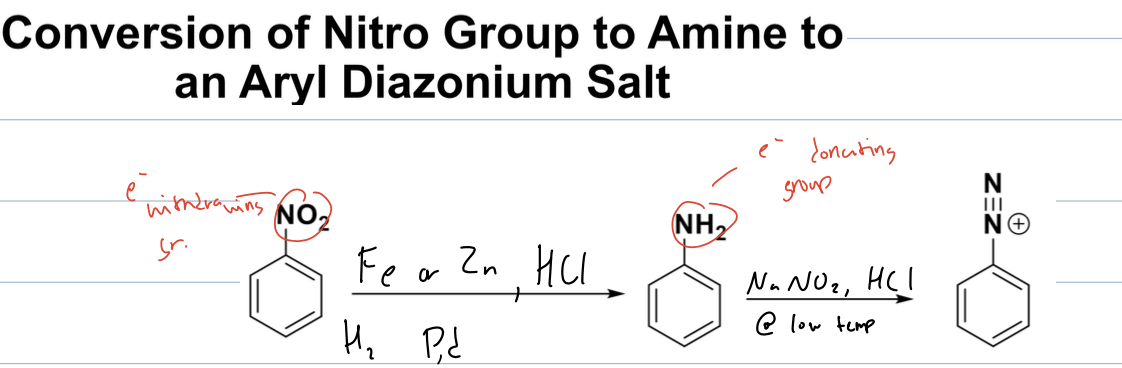
what rxn with nitrobenzene and Fe or ZN, HCL, H2, and Pd
then with that product and NaNO2, HCL, and low temp
conversion of nitrogroup to amine to an aryl diazonium salt
where the nitro group is changed to an amine
then use the aryl diazonium salt is formed when the amine is treated with NaNO2 and HCl at low temperatures, resulting in a stable diazonium compound.
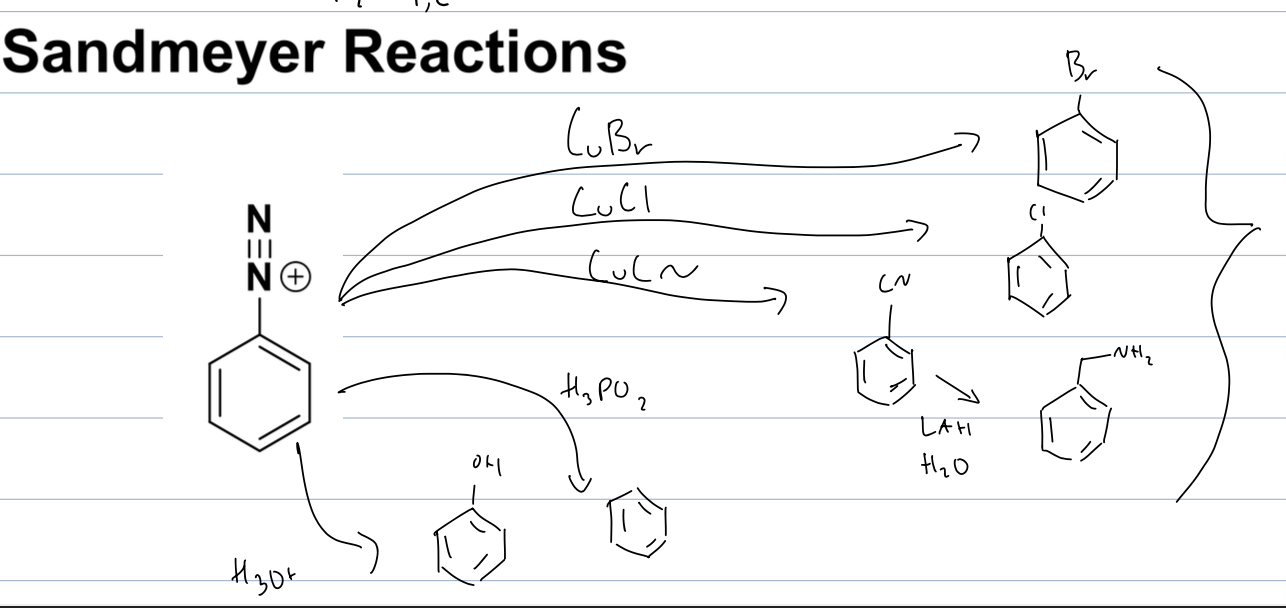
what rxn with diazonium salt and copper halide
sandmeyer rxn
where the diazonium salt can be substituted with any halide when its with copper.
can use LAH, H2O to change CN to a benzylic amine
can also use H3PO2 to remove diazonium salt
can use H3O+ to substitute with OH
what rxn with methyl chloride and AlCL3
friedel crafts alkylation
where the Cl attacks the AlCL3 and forms a carbocation that can add to the aromatic ring.
then the benzene attacks removing the methyl group to form sigma complex and then the ALCL4 deprotonates to restore aromaticity to the ring
can also use the same mech for an ethyl group
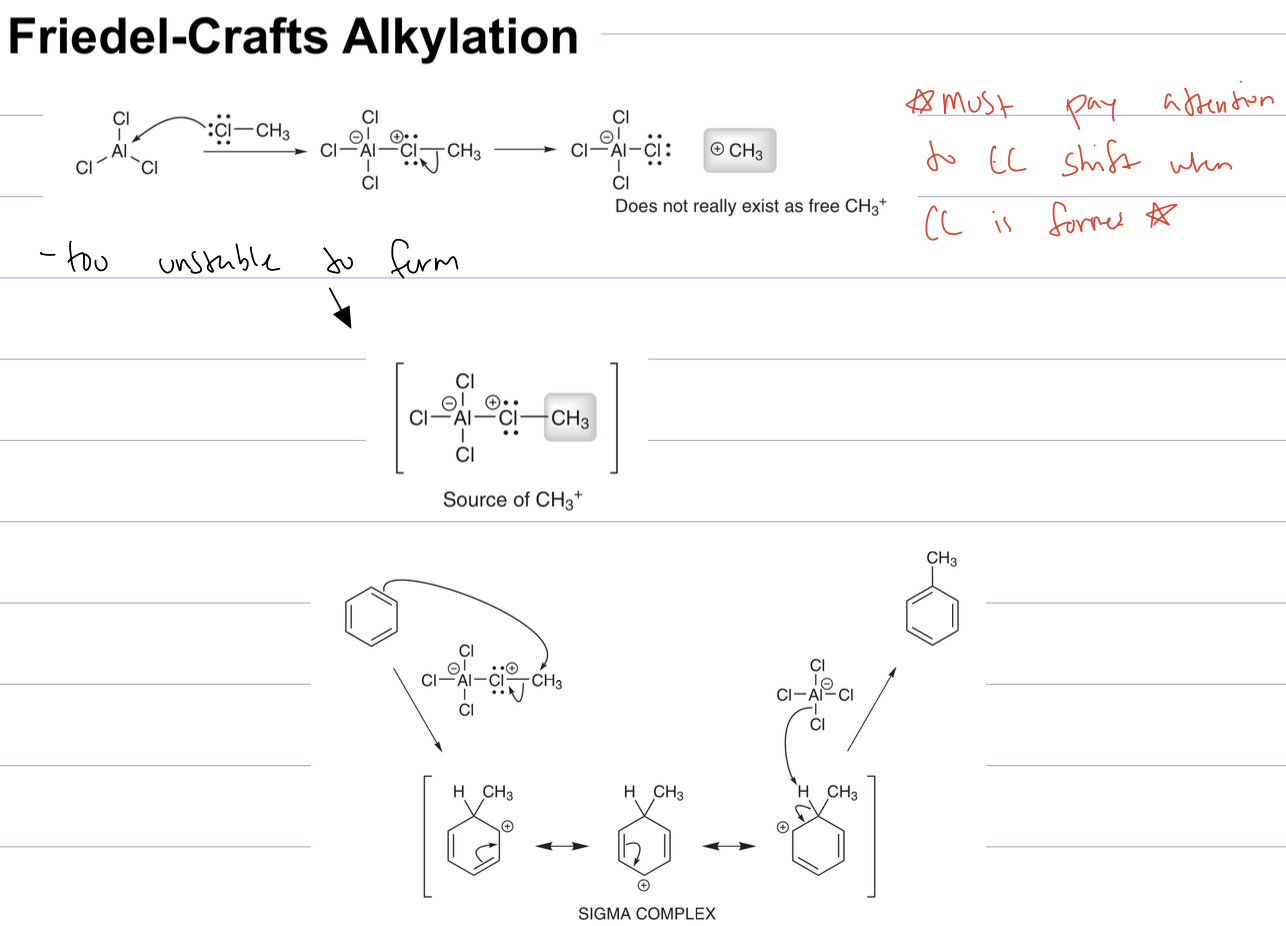
what is issue when using propyl group for friedel crafts alkylation
a CC shift can occur and yield a mix of products
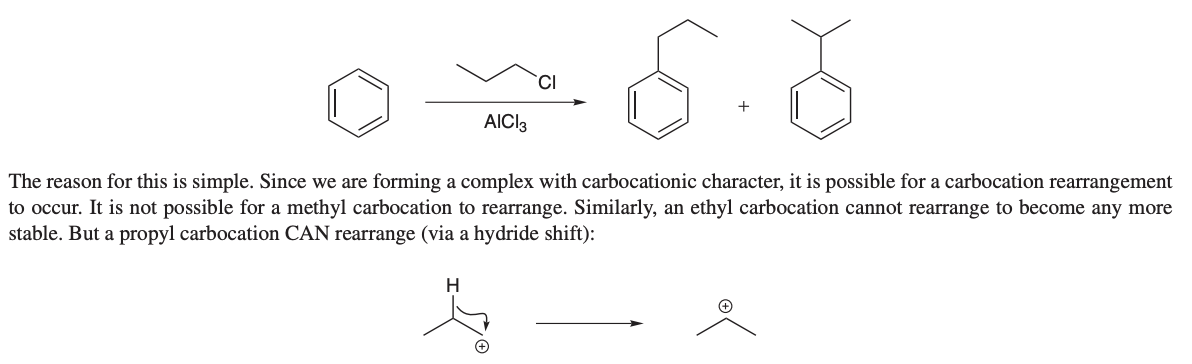
how do you instal alkyl group without a rearrangement
with reagents of an acyl chloride group and ALCl3, and Zn(Hg), HCL, heat
By using Friedel-Crafts acylation, which forms a ketone that can be reduced to the desired alkyl group.
where the Al attacks the other CL to form an acylium ion (which is resonance stabilized and wont rearrange), which then reacts with the aromatic ring to create a stable product without rearrangement.
the carbonyl is then removed with Zn(Hg) and HCL
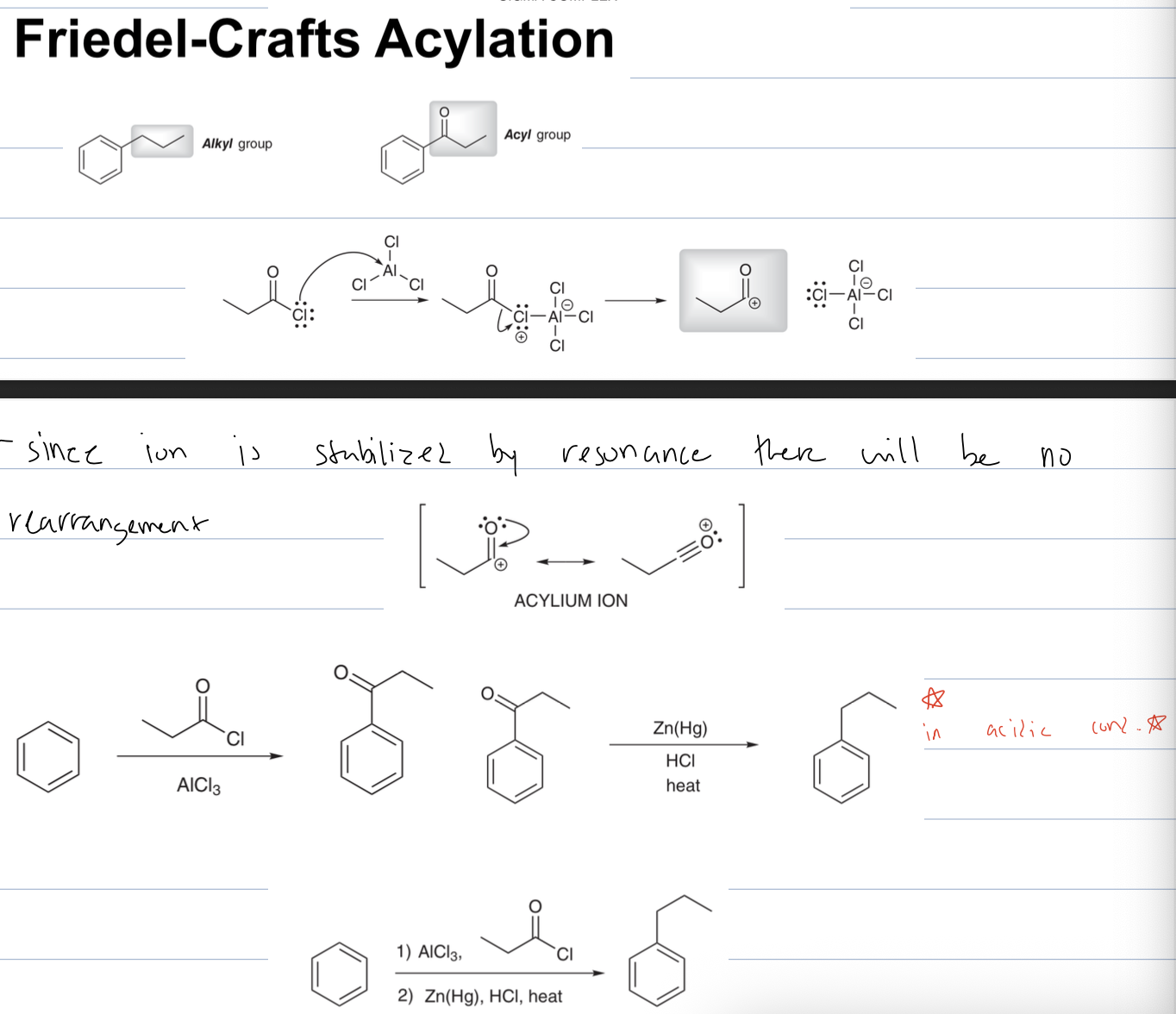
what is rxn to remove a carbonyl from an acyl group with Zn(Hg), HCL
clemmensen reduction
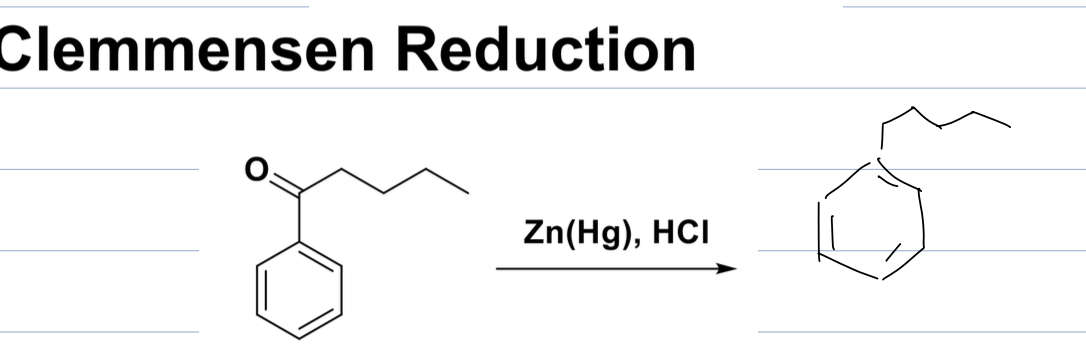
what is difference between alkylation and acylation with a propyl group
with alkylation you receive a mixture of products because of CC rearrangement
and with acylation you only get one product with an acyl group attached
the reagents are different too
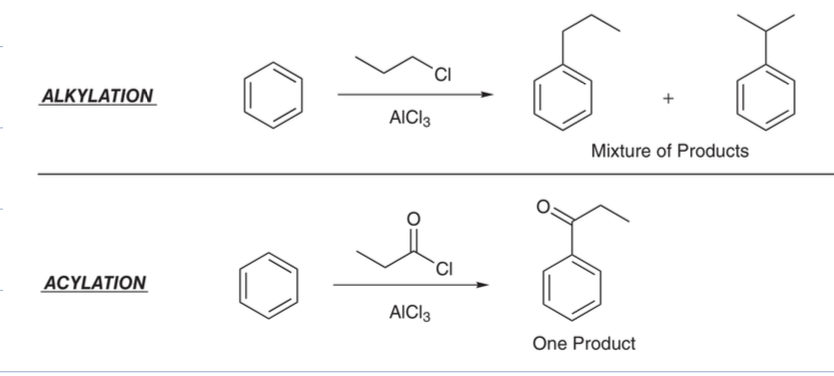
what are ortho, meta, and parapositions of a toluene
ortho is first two carbons
mets is bottom two carbons
para is opposite carbon
what is an activator
A substituent that donates electrons to the aromatic ring (creates a resonance structure with neg. partial charges), increasing its reactivity and favoring electrophilic substitution. and an ortho para director
what is a deactivator
A substituent that withdraws electrons from the aromatic ring (creating a resonance structure with part. pos charge), decreasing its reactivity and favoring meta substitution in electrophilic aromatic substitution reactions.
what are examlpes of activators on substituated benzene
strong activators
have lone pairs directly adjacent to the ring
moderate activators
have resonance outside the ring
weak
only have hyperconjugation
what is hyperconjugation (try and find better definition
Hyperconjugation is the stabilizing interaction where electrons in a σ-bond (usually C–H or C–C) delocalize into an adjacent empty or partially filled p-orbital (e.g., in a carbocation, radical, or alkene).
what is the halogen exception to activators and deactivators
The halogen exception refers to halogens acting as deactivators due to their electronegative nature, despite having lone pairs that might suggest activating behavior. In electrophilic aromatic substitution, they withdraw electron density from the ring, promoting ortho para substitution, rather than meta sub.
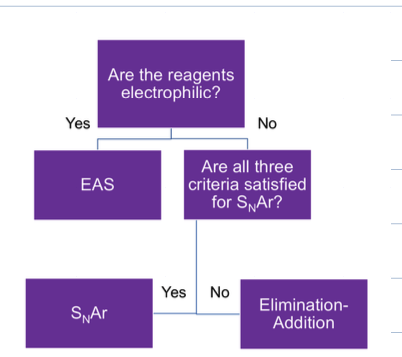
what is the criteria for NAS
The ring must contain a powerful electron withdrawing group (typicallly nitro)
the ring must contain a leaving group that is ortho or para to the electron withdrawing group, if meta no reaction is observed
what rxn with good EWS and Leaving group ortho or para and NaOH, 70c and H3O
NAS
where the leaving group is substituted with OH
the OH attacks where the LG is and the neg. charge kicks up into reservoir (EWS), then is moved around the ring through resonance making the meisenheimer complex. which results in the LG leaving and being substituted by OH
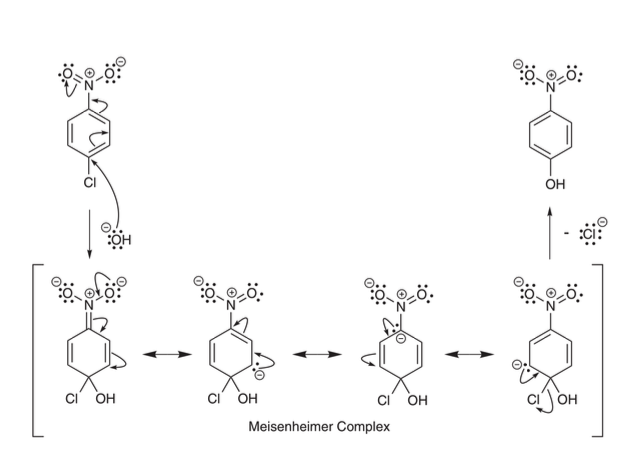
why must EWS be ortho or para to LG
otherwise the neg charge wont be able to be put onto reservoir to stabilize the intermediate during the nucleophilic aromatic substitution process.
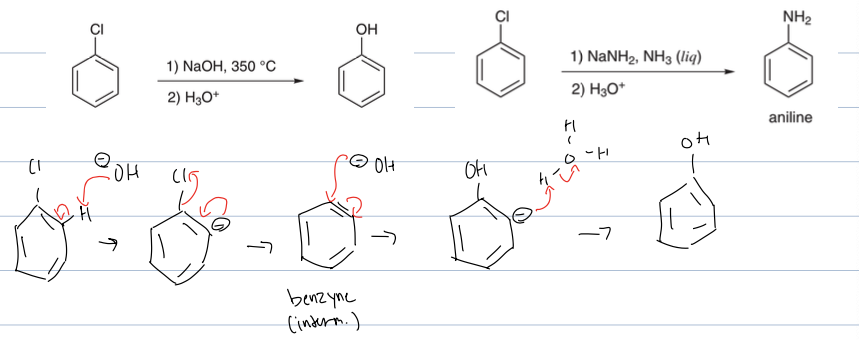
what is rxn with LG on benzene and NAOH, 350c, and H3O
Elimination addition
where the OH deprotonates allylic to leaving group putting a neg charge on that pos. then the neg charge hops onto double bond to create triple bond and the benzyne intermediate and the LG leaves. then the OH attacks where the LG was and kicking out triple bond back to double, and then it is worked up in acid to re protonate
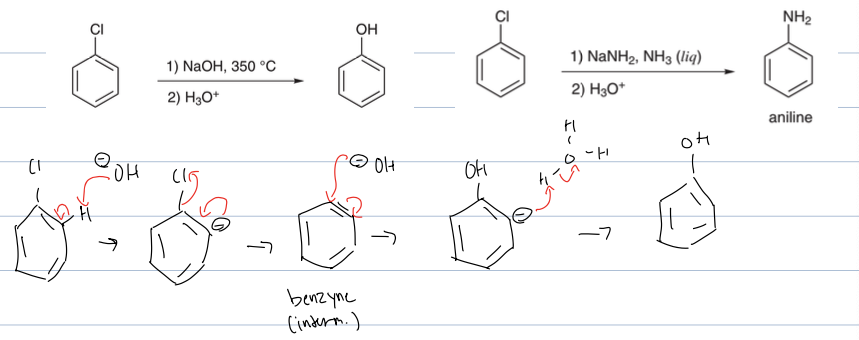
why is elim add under acidic conditions
because it needs to reprotonate after neg charge leaves benzyne intermediate
what is rxn with LG on benzene and NaNh2, Nh3(l) and H3O
Elimination addition
where the NH2 deprotonates allylic to leaving group putting a neg charge on that position. then the neg charge hops onto double bond to create triple bond and the benzyne intermediate and the LG leaves. then the NH2 attacks where the LG was and kicking out triple bond back to double, and then it is worked up in acid to re protonate
what is the flow chart of aromatic substitution rxns
what rxn with chromic acid, H2So4, H20 with a benzylic benzene and a proton on the benzylic pos.
oxidation to a carboxylic acid
must have protons in order to occur

what is rxn with benzylic benzene and NBS, Hv or heat
radical bromination
where BR is atted at most sub.
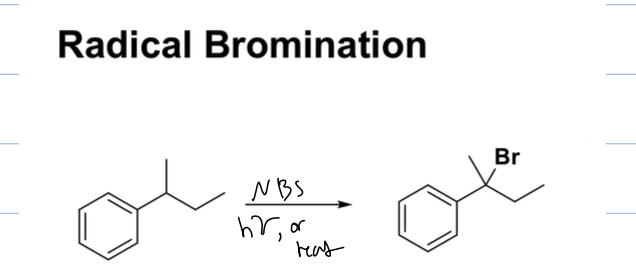
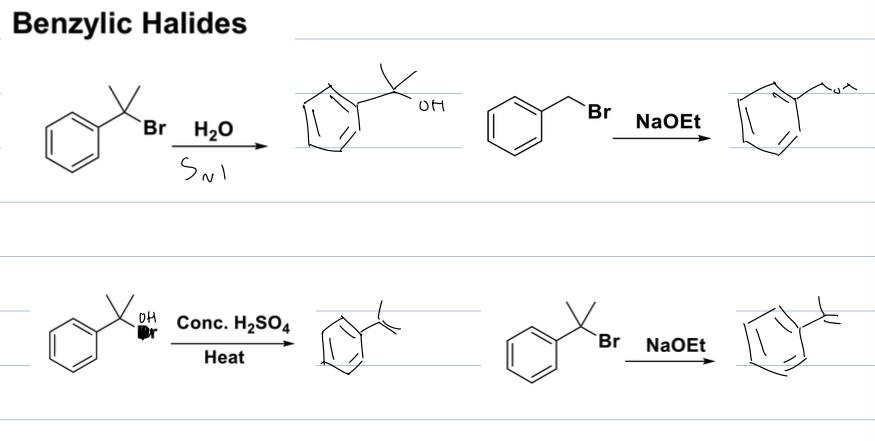
what rxns can you have with benzylic halides
subsitution and elim rxns
depends on substrate, LG and reagents