Complex Injectable Formulations and Their Applications
1/189
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
190 Terms
Complex injectables
Formulations requiring special physicochemical properties.
Physicochemical properties
Characteristics like solubility, stability, and viscosity.
Solubility
Ability of a substance to dissolve in a solvent.
Stability
Resistance to degradation over time and conditions.
Viscosity
Thickness or resistance to flow of a liquid.
Excipients
Inactive substances used to aid drug formulation.
Stabilizers
Agents that enhance the stability of formulations.
Adverse reactions
Unintended harmful effects from drug administration.
Product quality
Consistency and reliability of pharmaceutical products.
Modified release
Controlled release of drug over time.
Parenteral products
Drugs delivered via injection, bypassing the digestive tract.
Long-Acting Injectable (LAI)
Injectables designed for prolonged therapeutic effect.
NDA pathway
New Drug Application process for FDA approval.
In Vitro Release
Testing drug release in a controlled environment.
Aqueous crystalline drug suspensions
Suspensions containing solid drug particles in liquid.
Aripiprazole
Antipsychotic medication used for schizophrenia treatment.
Abilify Maintena Kit
LAI formulation of aripiprazole for schizophrenia.
Medroxyprogesterone acetate
Hormonal drug used for contraception.
Depo-Provera
LAI contraceptive with 3-month efficacy.
Paliperidone palmitate
LAI antipsychotic for schizophrenia with extended release.
Invega Trinza
LAI formulation for schizophrenia with 3-month duration.
Approval date
Date when a drug receives regulatory approval.
Exenatide
Synthetic drug for Type 2 diabetes management.
Bydureon
Exenatide formulation, 2 mg weekly, SC administration.
Bydureon Pen
Pre-filled pen for Bydureon, 2 mg weekly, SC.
Bydureon Bsice
Bydureon variant, 2 mg in 0.85 mL, SC.
Leuprolide acetate
Hormone therapy for prostatic cancer treatment.
Lupron Depot
Leuprolide formulation, 3.75 mg monthly, IM.
Lupron Depot-Ped
Leuprolide for central precocious puberty, IM.
Minocycline HCl
Antibiotic for periodontitis, 1 mg base variable.
Naltrexone
Medication for alcohol dependence, 380 mg monthly.
Octreotide acetate
Acromegaly treatment, varying doses every four weeks.
Pasireotide pamoate
Acromegaly and Cushing's disease treatment, IM.
Risperidone
Antipsychotic for schizophrenia, varying doses, IM.
Triamcinolone acetonide
Osteoarthritis pain relief, 32 mg every 3 months.
Triptorelin pamoate
Prostate cancer treatment, varying doses, IM.
Triptodur Kit
Central precocious puberty treatment, 22.5 mg, IM.
Buprenorphine
Opioid disorder treatment, 100 mg/0.5 ml monthly.
Degarelix acetate
Prostate cancer treatment, 80 mg every 28 days.
Doxycycline hyclate
Antibiotic for periodontal disease, 50 mg subgingival.
Lanreotide acetate
Acromegaly treatment, varying doses every 4 weeks.
Goserelin acetate
Prostatic cancer treatment, 3.6 mg every 28 days.
Modified injectables
Insoluble drugs in emulsion for local effect.
Modified release injectables
Sustained release for chronic administration, reduced frequency.
Long-acting injectables (LAIs)
Sustained drug release for prolonged therapeutic effect.
Sustained-release formulation
Delivers drugs over extended periods, enhancing efficacy.
Injection frequency
Reduced number of injections improves patient adherence.
Cost-effectiveness
Lower dosing reduces overall drug expenses.
Therapeutic efficacy
Increased effectiveness of treatment through optimized delivery.
Dose dump
Premature drug release leading to potential overdose.
Intramuscular (IM) injection
Administers 2-5 ml into large muscle groups.
Subcutaneous (SC) injection
Administers 1-2 ml into fatty tissue.
Immunogenicity
Ability of substances to provoke immune responses.
Cyclodextrins
Carbohydrates forming complexes to enhance drug solubility.
Inclusion complex
Complex where drug is encapsulated within cyclodextrin.
Hydrophilic outer surface
Water-attracting exterior of cyclodextrin molecules.
Lipophilic central cavity
Fat-attracting interior of cyclodextrin for drug storage.
FDA approved derivatives
SBE-ß-CD and HP-ß-CD for parenteral use.
Long-term toxicity
Assessment of prolonged drug exposure effects.
Antigenic substances
Molecules capable of triggering immune reactions.
IgG1 levels
Immunoglobulin response measured after protein administration.
Prolonged release benefits
Useful for diabetes, cancer, contraception, osteoporosis, arthritis.
Volume of IM injection
Typically between 2-5 ml for effective delivery.
Volume of SC injection
Typically between 1-2 ml for effective delivery.
Safety in injections
Avoiding veins/arteries requires trained personnel.
Molecular rigidity
Structural inflexibility affecting cyclodextrin solubility.
Hydrogen bonding
Intermolecular forces impacting cyclodextrin crystal state.
Hydrogen Bond
Attraction between hydrogen and electronegative atoms.
βCD
Beta-cyclodextrin, has complete secondary belt structure.
Solubility of βCD
Approximately 18 mg/ml in water.
Sulfobutyl Ether βCD
Modified βCD with solubility over 1200 mg/ml.
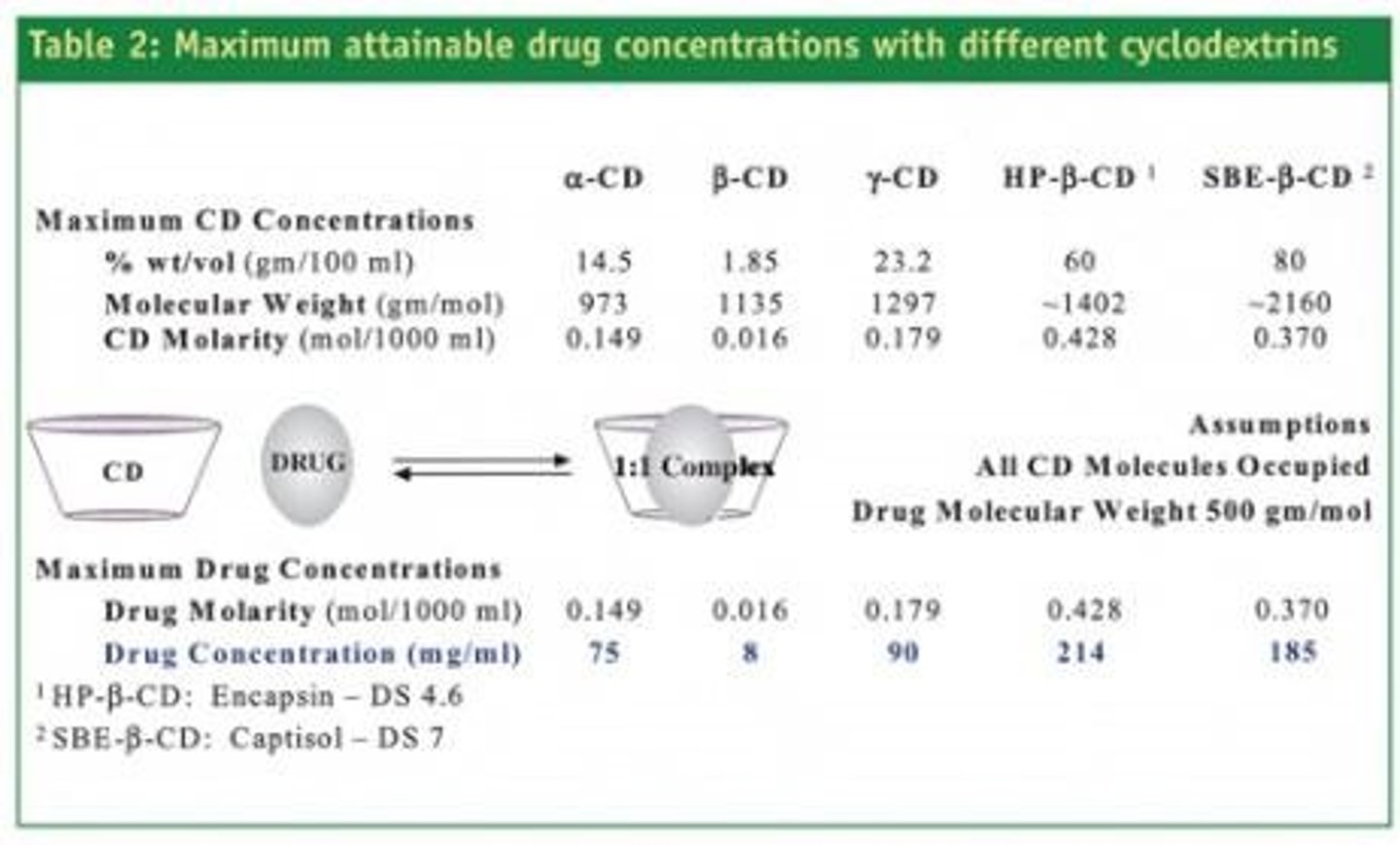
αCD
Alpha-cyclodextrin, has incomplete hydrogen bond belt.
γCD
Gamma-cyclodextrin, features non-coplanar structure.
Cyclodextrin Advantages
Improves solubility, bioavailability, and stability.
Tissue Irritation Reduction
Minimizes discomfort from drug administration.
Odor Masking
Conceals unpleasant smells of drugs.
Voriconazole
Antifungal drug using SBE7-β-CD for IV administration.
Ziprasidone
Antipsychotic drug using SBE7-β-CD for IM administration.
Aripiprazole
Antipsychotic drug using SBE7-β-CD for IM administration.
Telavancin
Infection treatment using HP5-β-CD for IV administration.
Oily Vehicles
Used for modified release parenteral products.
Isopropyl Myristate
Synthetic fatty acid ester alternative vehicle.
Ricinoleic Acid
High content fatty acid in castor oil.
Esterification
Process to create long-chain fatty acid drug derivatives.
Hydrolysis
Chemical breakdown of esters to release parent drug.
Autoxidation
Degradation of unsaturated fatty acids by heat/light.
Oil/Water Partition Coefficient
Influences drug absorption rate in formulations.
Aqueous Suspension
Saturated solution with suspended drug particulates.
Paliperidone Palmitate
Prodrug formulation of paliperidone for IM injection.
Invega Sustenna
Injection formulation given every 28 days.
Testosterone Undecanoate
Long-acting depot formulation in castor oil.
Log P
Measure of drug's hydrophobicity; higher means less soluble.
Therapeutic Action Duration
Lasts from one week to one month.
CABENUVA
Cabotegravir and rilpivirine injectable suspension for HIV.
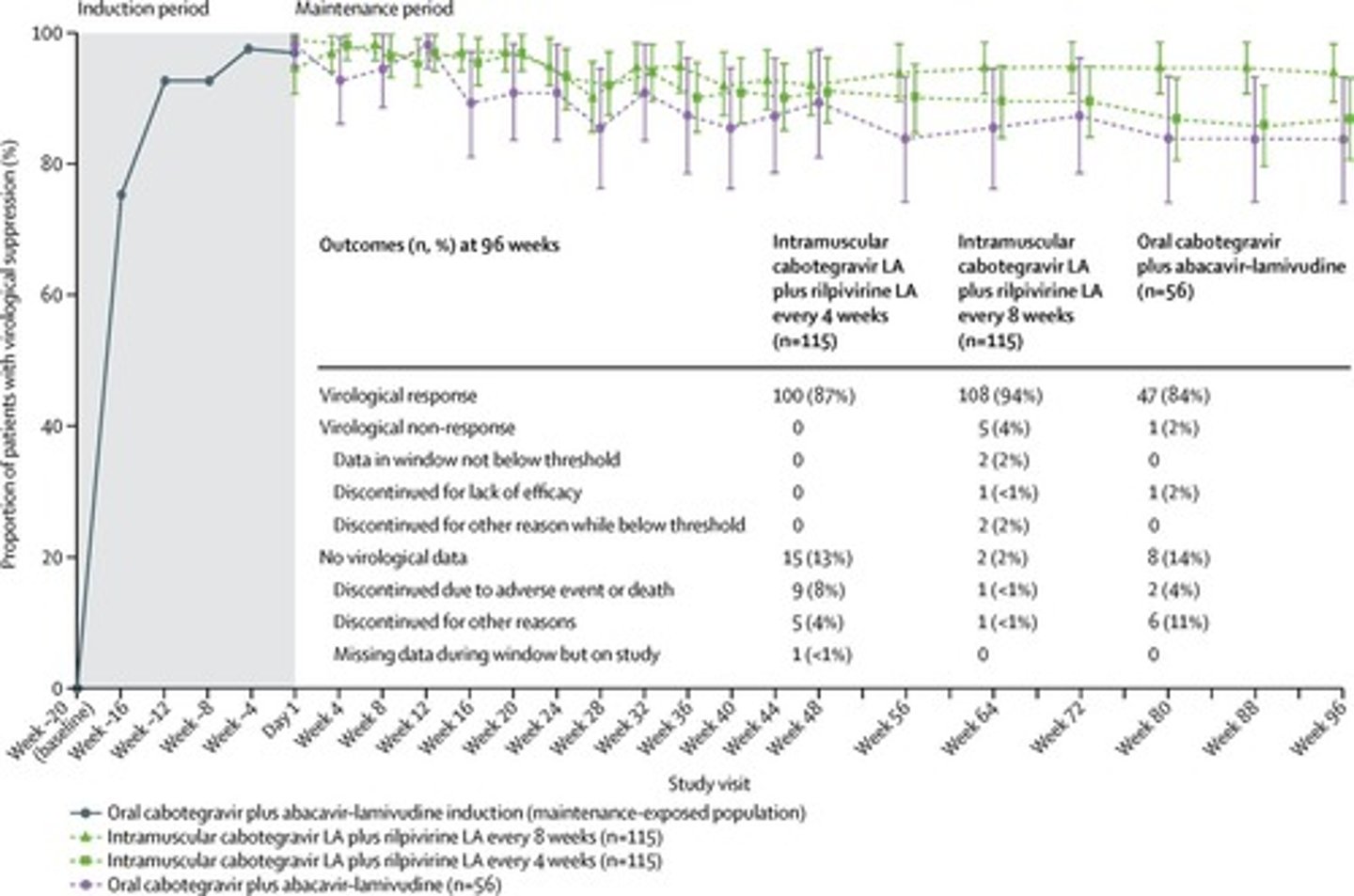
Initial Approval
First U.S. approval in 2021 for CABENUVA.
HIV-1 RNA Concentration
Measured in copies per mL; targets <50 copies.
FDA Snapshot Algorithm
Method to assess treatment outcomes in HIV patients.
Formulation Parameters
Factors affecting therapeutic benefit of drug formulations.
Particle Size
Influences drug absorption and therapeutic efficacy.
Crystal Habit
Refers to the geometric shape of drug crystals.