dev bio exam 3 clean
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
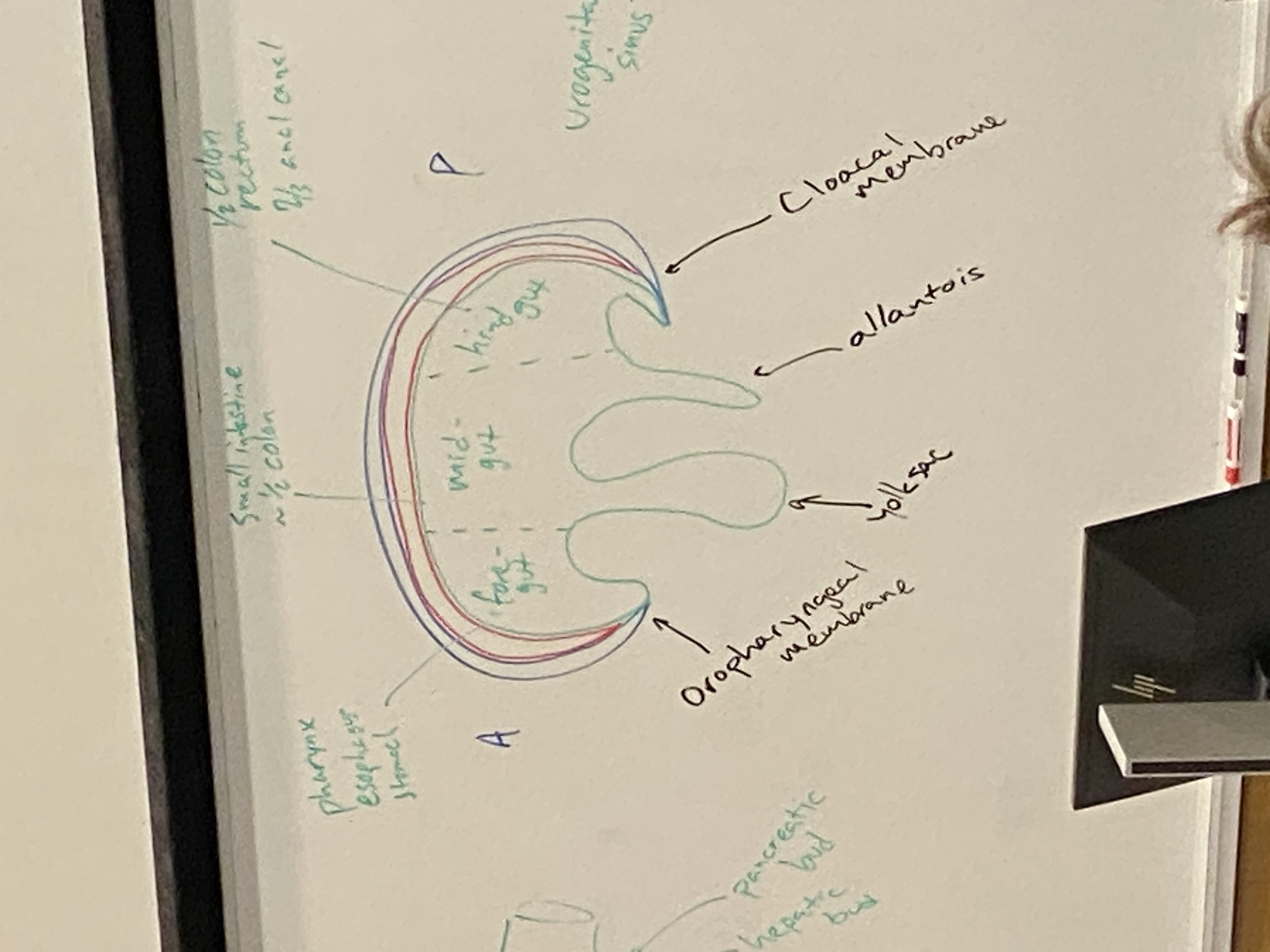
what are the mesoderm-free membranes at the cranial and caudal ends of the embryo, and what will they subsequently become?
oropharyngeal and cloacal membrane; become two openings to digestive system
what are the three sections of the primitive gut, and what parts of the alimentary canal do they become?
foregut → pharynx, esophagus, stomach, and ~1/2 of duodenum. also the respiratory bud (lungs), hepatic bud (liver), and pancreatic bud (pancreas)
midgut → lower half of duodenum, small intestine, ~1/2 of the colon
hindgut → second half of colon, rectum, ~2/3 of anal canal. urogenital sinus → bladder, urethra, prostate, birth canal
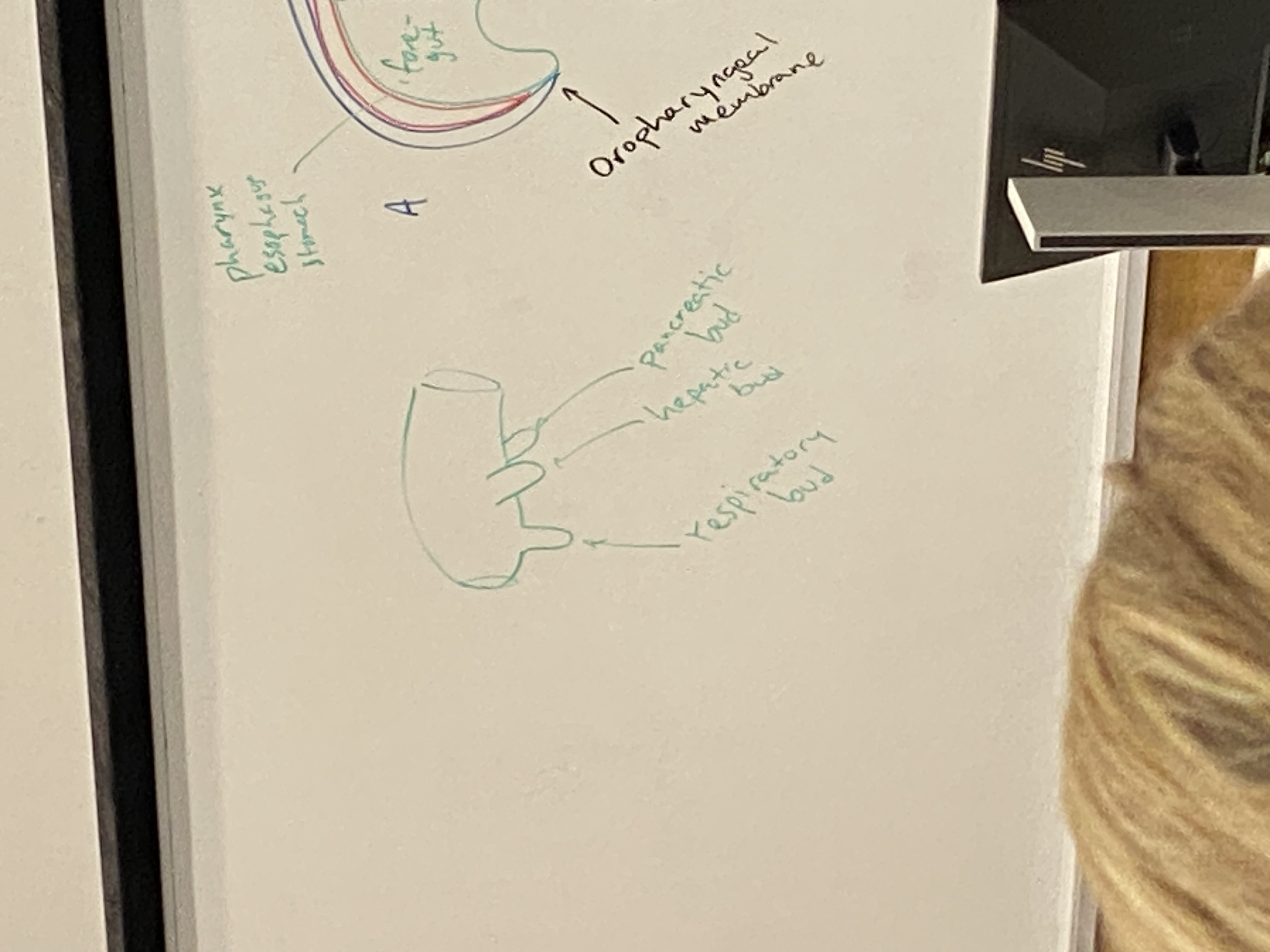
what are the three buds that emerge from the foregut and what do they become?
posterior: pancreatic bud. becomes pancreas and its ducts
side-ish: hepatic bud. becomes liver and gallbladder and their ducts
most anterior: respiratory bud. becomes lungs
why is it thought that lungs are evolutionarily derived from the swim bladders of ancient fishes?
frozen accident of evolution: our ancestors were fish that breathed thru gills. they have gas bladders, an outpocket of the digestive system, used for buoyancy. later descendants had more and more convoluted gas bladders that became more and more connected to the circulatory system → lungs
this is why our respiratory and digestive systems are connected, hence why swallowing can be dangerous
draw the gene regulatory network diagram that leads to the indifferent gonads differentiating into either ovaries or testes
XX → Wnt4 → beta-catenin → Fox2 →→→ ovary determining factors (ODF) which actually cause the gonads to differentiate
XY → SRY → Sox9 →→→ testes determining factors
Sox9 ←→ Fgf9
ALSO note that beta catenin and sox mutually inhibit each other!!
once the above process (differentiation of gonads into ovaries or testes) has occurred, what are the key cells and hormones that contribute to the differentiation of the internal/external reproductive structures?
male: leydig cells → testosterone → 1) maintenance of wolffian ducts; 2) dihydrotestosterone (DHT) → external genitalia
sertoli cells → anti-mullerian hormone (AMH) → active degeneration of mullerian ducts
female: no testosterone → degeneration of wolffian ducts
follicular cells → estrogen → internal and external genitalia

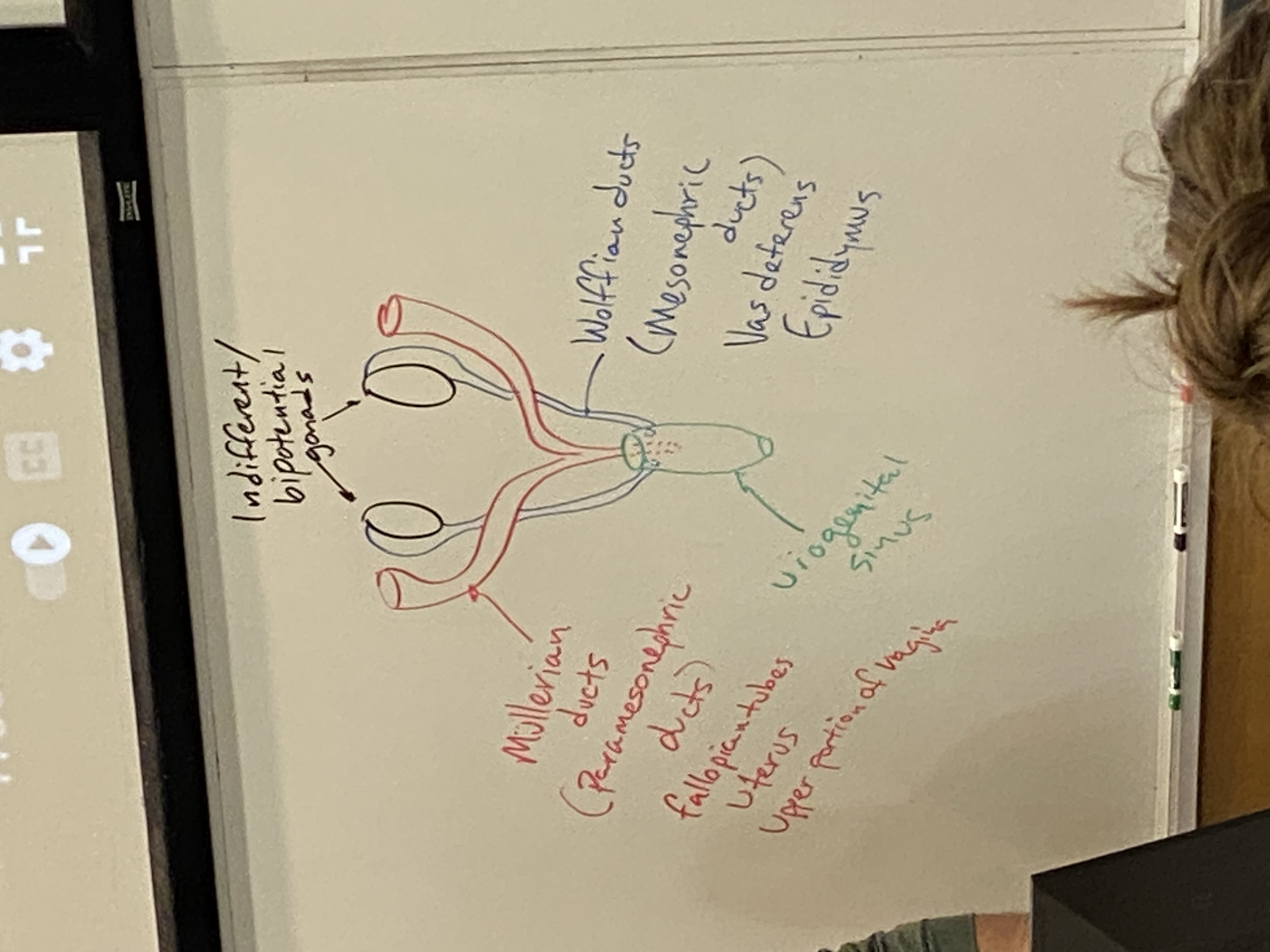
draw and label the internal reproductive structures as they appear at the bipotential/indifferent stage
female red + labelled on left; male in blue + labelled on right
how do the germ cells end up in the indifferent gonads?
primitive streak, primitive groove, embed into yolk sac/hindgut, then at certain point when gonadal ridge is forming they hike up thru gut tube, cross dorsal mesentery, embed into indifferent gonads.
sequestered away from other signalling so they remain in pleuripotent state; no decisions about their identities get made prematurely
from what germ layers are the various reproductive/urinary structures derived?
gonads come from intermediate mesoderm → wolffian and mullerian ducts
mullerian ducts → fallopian tubes, uterus, cervix, upper birth canal; thus these are all mesodermal
hindgut differentiates into two pathways. front becomes urogenital sinus → bladder, urethra, prostate, and bottom 2/3 of the birth canal; thus these are all endoderm
very end of male urethra comes from invagination of surface ectoderm
what genes are involved in differentiating fore- and hind-limb identity?
Tbx5 (forelimb)
Tbx4 and Pitx1 (hindlimb)
what are the generalized terms for the sections of vertebrate limbs along the proximo-distal axis and which hox genes specify their fates?
stylopod, zeugopod, autopod
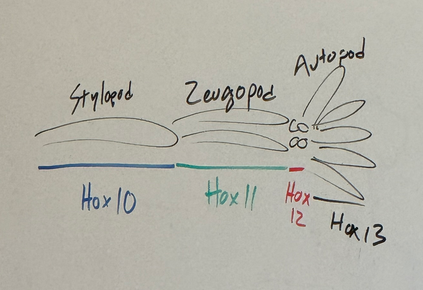
in addition to hox genes, what other signaling factors are responsible for regulating proximo-distal axis formation?
fgf8, fgf10, wnt3A, Shh
Hox13 (distal end — digits) inhibits Wnt3A — terminates lengthening
Shh is excreted in limb bud from ZPA (zone of polarizing activity — determines a-p axis) to drive fgf10 expression in progress zone. fgf10 and fgf8 induce each other. fgf8 and wnt3a induce each other.
fgf8 is primarily responsible for the mitosing and continued growth distally; the heart of progress. sourced from apical ectodermal ridge
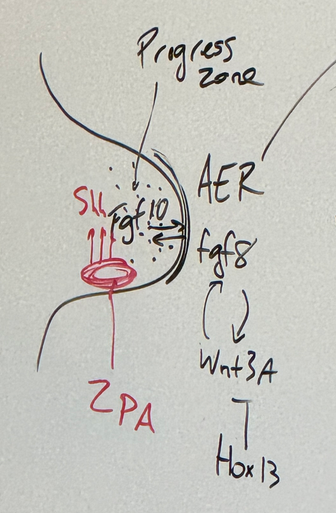
draw a diagram of the developing limb bud that includes the three major signaling/organizing centers
how is anterior-posterior digit identity determined and what happens when an ectopic anterior duplication of this signaling center occurs (either experimentally or mutationally)
zone of polarizing activity (ZPA) excretes Shh — gradient tells each digit which it’s supposed to be. the posterior region (closer to ZPA) has higher express → pinky. further away (anterior) has basically no Shh → thumb
an ectopic anterior duplication is caused by having ZPA in the anterior portion as well; leads to mirror hand syndrome
fingers are separated by BMP expression which causes apoptosis. in bat wings/webbed feet, the gene gremlin inhibits BMP so the digits are connected.
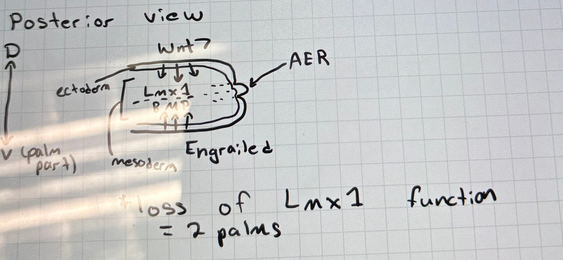
what key genes pattern dorsal-ventral limb identity
dorsal (back of hand): ectoderm wnt7 → Lmx1
ventral (palm): mesoderm engrailed → BMP
loss of Lmx1 = 2 palms
what key gene is consistently required for the formation of photo-sensitive cells across the animal kingdom, and what does this knowledge tell us about the common ancestor of bilateria?
pax6
the common ancestor of bilateria could sense light/dark, but did not have true eyes yet — explains how vertebrates and mollusks have different eyes
from an evolutionary perspective, what is the use in having “half an eye?”
each development in eye structure gives a little more help — go from seeing just light/dark → directionality → poor image (pinhole style — eclipse glasses) → focused image (true camera-style)
how do we know that mollusk eyes and vertebrate eyes evolved independently, despite having similar architecture?
wired differently!
vertebrates — neural connection to photoreceptors — “wires” that feed them go in front of the photoreceptors and also have to all bundle up and pass through to connect to nervous system — blindspot
mollusks have no blindspot because the photoreceptor cells are wire from the back.
thus the common ancestor of vertebrates and mollusks had no eyes
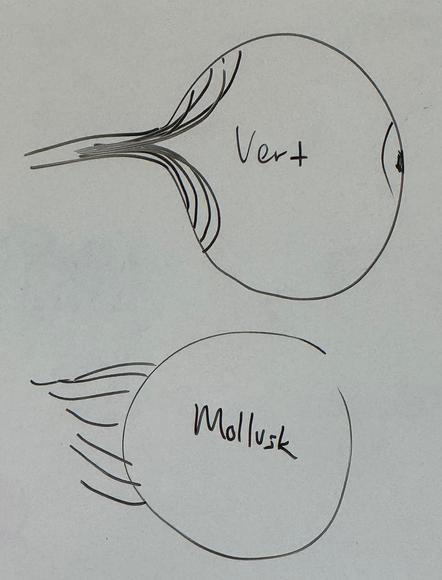
what leads to the induction and separation of the two eye fields?
Shh turns off pax6. lead to a LOT of cell division in neural tube ventrally → pax6 expression on the borders (where Shh is not active) turns into two eye fields moving apart from each other.
draw the stages of eye induction through the formation of the lens vesicle
step 1
neural tube branch is going to smoosh up against surface ectoderm. called the optic vesicle.
fgf signalling back and forth at this junction
lens placode forms.
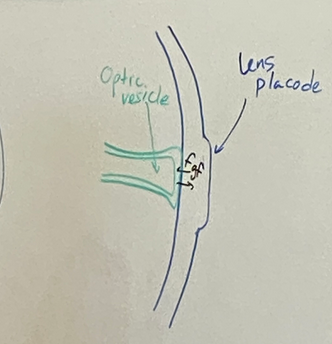
draw the stages of eye induction through the formation of the lens vesicle
step 2
invagination after lens placode development
optic vesicle becomes optic stalk which will be the optic nerve eventually
optic cup and lens pit form at the interface between the neural tube and surface ectoderm
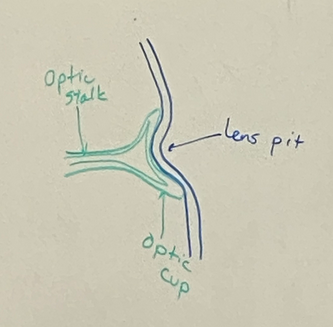
draw the stages of eye induction through the formation of the lens vesicle
step 3
two most ventral parts of surface ectoderm join up together
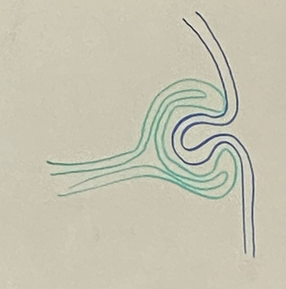
draw the stages of eye induction through the formation of the lens vesicle
step 4
now our eye is a hollow ball of cells almost totally surrounded by neural tissue, but if we were to look up from the bottom (drawing in top left), we would see a little canal/opening for blood supply — a single artery and a single vein (hyaloid vein and artery). this fissure is the choroidal fissure — temporary structure just while lens vesicle is still made of cells that need nutrients. cross section is shown at bottom left
lens vesicle forms. cells will eventually crystallize and lose their organelles and fill with cristalin
surface ectoderm does more invaginating

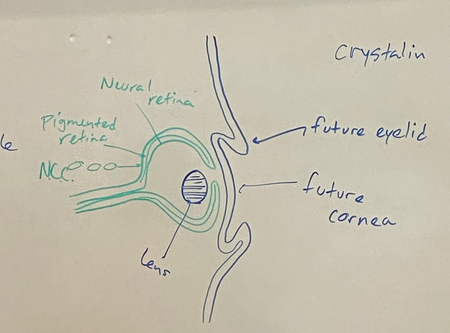
draw the stages of eye induction through the formation of the lens vesicle
step 5
eventually we will get the eyelids from the surface ectoderm later in fetal development
lens will be filled with thin fibers primarilly made of the protein cristalin, which makes it clear
cornea is derived from surface ectoderm
two layers of retina — neural retina (front layer) and pigmented retina (back layer). vertebrate eyes have blindspots because the nerves are in the front, so the light has to pass thru the nerves to hit the photosensitive cells, and all these nerves have to go thru one hole for the optic nerve. the pigmented retina
neural crest cells invade and make melanocytes in the back of the retina so they can better absorb light. don’t want a lightly colored one bc the reflected light would bounce all around and eyes wouldn’t work too good
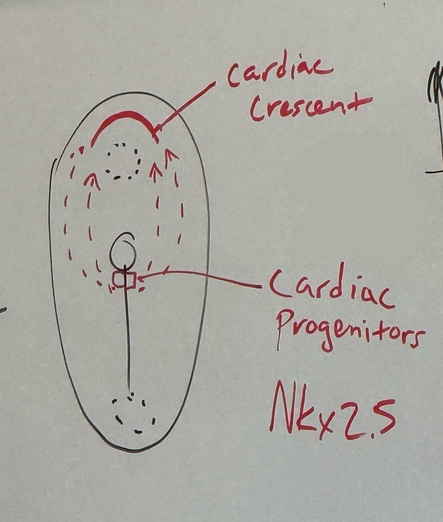
what key gene is consistently required for the formation of cardiac tissue across the animal kingdom, and what does this knowledge tell us about the common ancestor of bilateria?
Nkx2.5/2-5 is a master regulatory switch gene. super highly conserved across all animals. we know that common ancestor of all bilateria had a heart that probably looked relatively similar to modern hearts
from where do the cardiac progenitors initially originate and to where do they migrate?
cells of mesoderm, basically immediately posterior to primitive node — cardiac progenitors
gene expression differentiates them into these early cardiac cells — Nkx2-5 is the master regulator for all things that have a heart. in drosophila, known as tinman.
cardiac progenitor cells start diving into primitive groove and march themselves way to the anterior of the embryo, past the oropharyngeal membrane to form a temporary structure called the cardiac crescent
(the cardiac crescent cells then form first heart tube — trilaminar disc. then lateral and cranial-caudal folding of trilaminar disc. bring the parts together and fuse to create thicker, shorter primitive heart tube that will hook up to circulatory system and become functional)
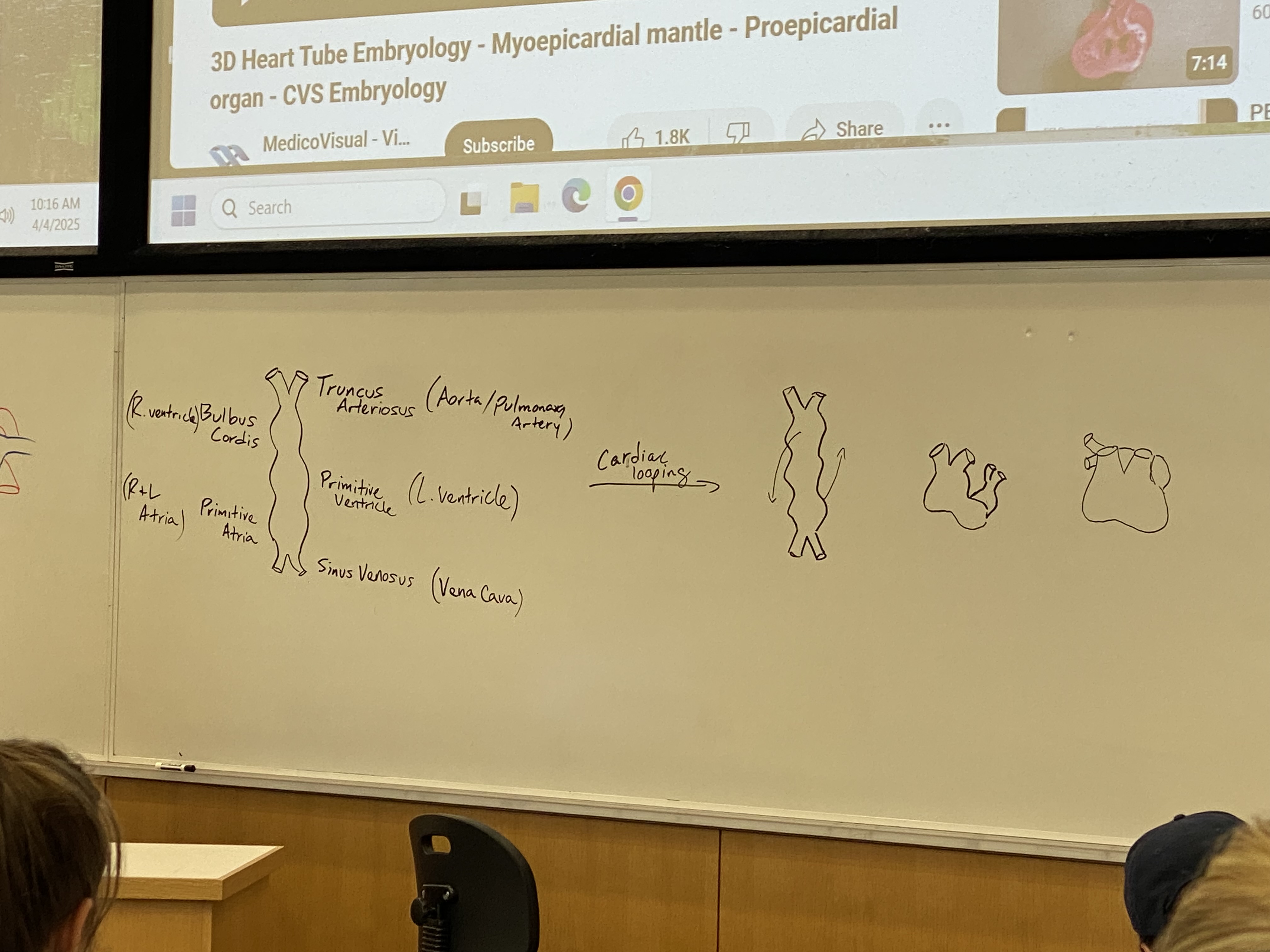
draw and label the features of the primitive, differentiated “heart tube”, with flow in from bottom and out from top (^)
outflow tubes: truncus arteriosus becomes aorta/pulmonary artery
bulbus cordis becomes R ventricle
primitive ventricle becomes L ventricle
primitive atria becomes R+L atria
inflow tubes: sinus venosus becomes vena cava

what is cardiac looping?
top part w ventricles starts looping to (its right, my left) coming out at me + down
atria part starts doing the opposite, looping to (its left, my right) going back into the board + up
process continues until primitive atria loops behind, up, and back to rest on top of the primitive ventricle + bulbus cordis
all of the chambers will fuse again so we will have one big empty heart sac; chambers will have to be built after
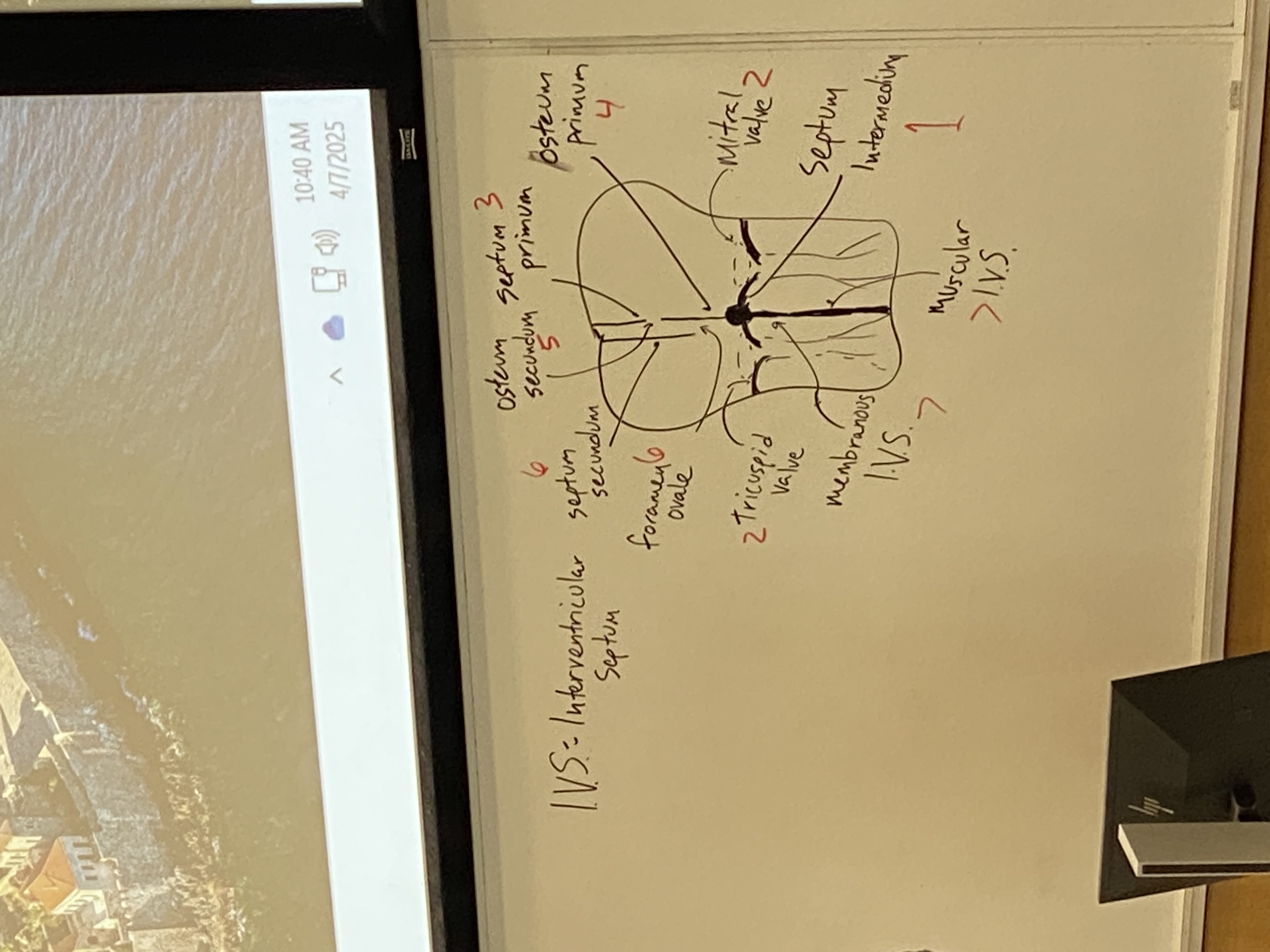
draw and label the structures of the embryonic heart after cardiac looping is complete and the atrioventricular valves and septa have formed
note that the osteum primum is not drawn, it should be a gap at bottom of septum primum just before reaching the septum intermedium
see bar of tissue bisecting from front to back of heart. in this section, it looks like a dot. called the septum intermedium. serves as organizing center for dividing atria and ventricles (top and bottom; left and right)
flaps differentiate out from septum intermedium. additional ones assemble on either side as well. atrioventricular valves. some tendons also form from this to prevent backflow of the valves
mitral valve, tricuspid valve separate the atria from the ventricles
septum primum doesn’t initially seal off at the bottom but will eventually close that gap (osteum primum) to the septum intermedium, but at the same time a new gap opens up superiorly (osteum secundum)
septum secundum will form to somewhat block off the osteum secundum but not entirely — leaves an opening. this channel is the foramen ovale
membranous portion I.V.S. (of the intraventricular septum) and muscular I.V.S.
we now have both atria and ventricles
what are the functions of the foramen ovale and ductus arteriosus?
foramen ovale: to pump/funnel blood away from the lungs bc they’re basically useless in this moment — no gas exchange bc the baby isn’t breathing air
ductus arteriosis: connection between pulmonary artery and lungs so even more of mom’s oxygenated blood from r ventricle gets shoved out to aorta to go to rest of body
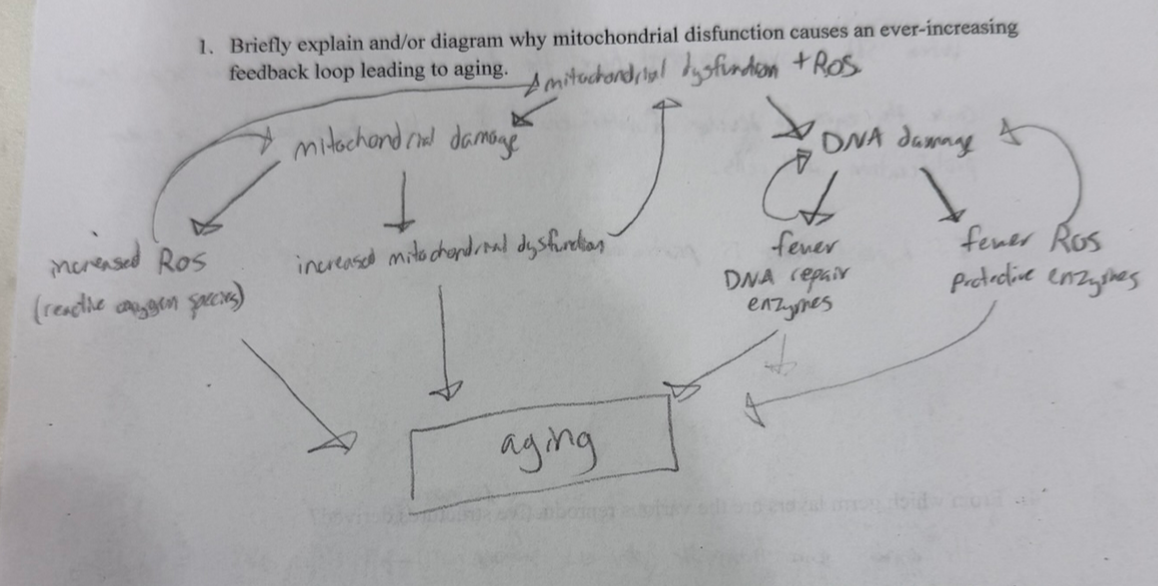
how does mitochondrial dysfunction contribute to aging?
mitochondrial damage → incr mit dysfunction and incr ROS
DNA damage → fewer DNA repair enzymes and fewer ROS protective enzymes
they both exacerbate each other!
what evidence suggests that patterns of chromosomal methylation become increasingly haphazard with age and why might that cause transcriptional “noise” in older individuals?
stain chromosomes of two twins. overlap of genes is yellow. if you look at 3 yr olds, see lots of overlap. in older twins, very little yellow, meaning that methylation over life is getting more haphazard. if you have haphazard methylation, gene expression patterns change in weirder and weirder ways as you age, i.e. silencing repair enzymes → incr DNA damage. nasty positive feedback loops, as these are partially caused by ROS and DNA damage and mutations to the methyltransferase enzymes
why might caloric restriction be associated with increased longevity?
IGF-R1 → PKB —| foxO
eat a bunch of calories, a bunch of IGF-R1 is expressed, a bunch of PKB expressed, foxO is INACTIVE. phase of DNA damage
restrict caloric intake, foxO is on by default, we go into repair mode (DNA repair and ROS protection)
how might an idiosyncrasy of linear chromosomal replication contribute to aging?
need 3’ OH end for DNA pol, so need RNA primer that will need to be replaced. euks can’t replicate those ends bc there’s no way to add/remove primers how we normally would. every time they replicate, the telomeres shrink (telomere attrition). eventually get too short and start eating the useful information of the chromosome.
genital ridge
thickening of intermediate mesoderm that will eventually become gonads
primordial germ cells are embedded ___ and migrate up to the ___ to embed in the ___
embedded back somewhere in the yolk sac and/or hindgut
migrate to genital ridge to embed in the (currently) bipotential gonads
if you are missing any gene function in the XY pathway, what will you turn into?
an anatomical female with XY karyotype
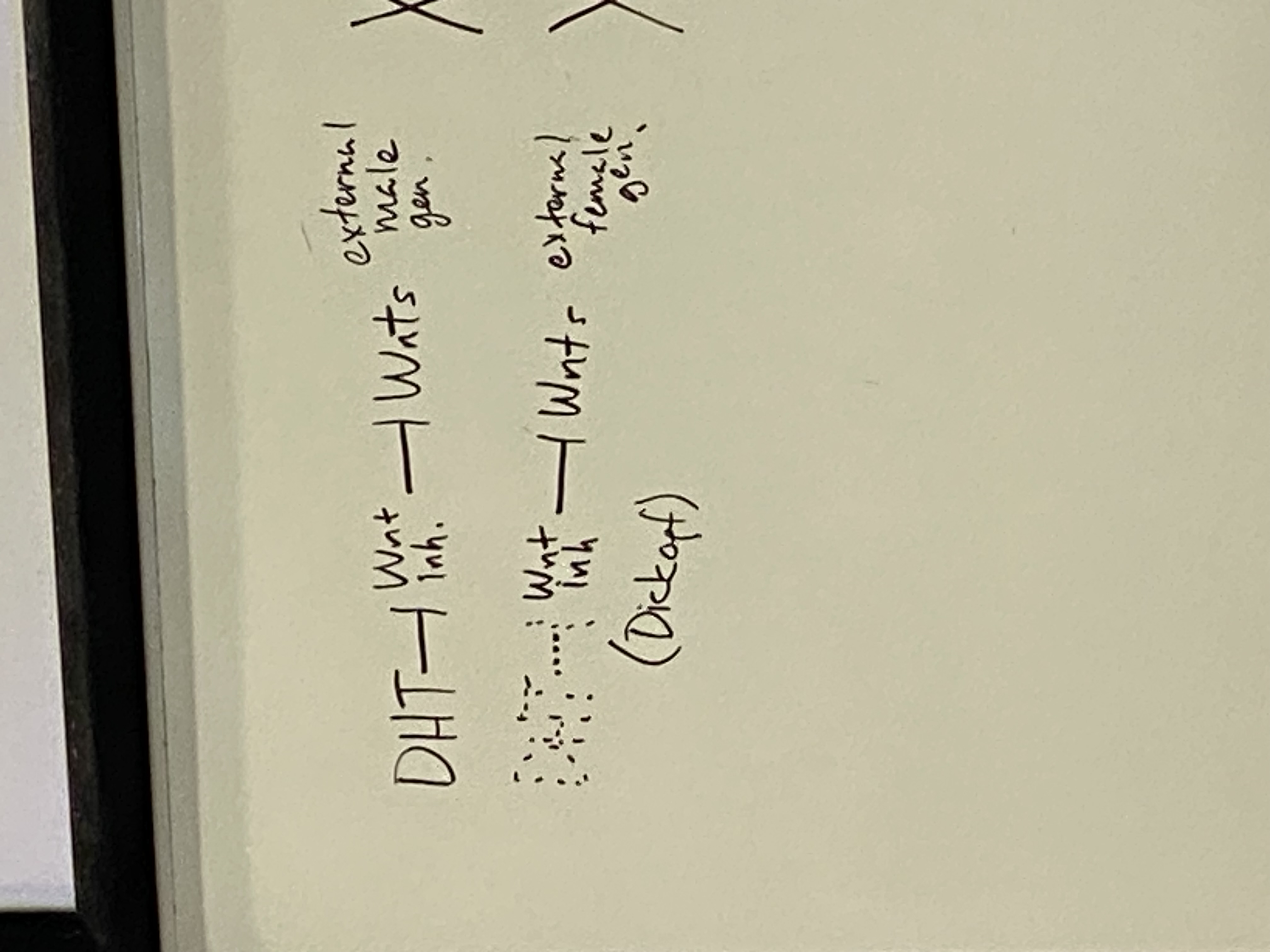
DHT is inhibitor of Wnt inhibitors — thus DHT is an indirect activator of Wnt
are Wnts activated in males or females?
Wnts active in males (they have dihydrotestosterone, so they inhibit the inhibitors of Wnt)
inactive in females
need to know what these various parts will become in male/female: genital tubercle, urogenital folds, labioscrotal swelling
genital tubercle: glans penis + erectile tissues; clitoris
urogenital folds: body of penis; labia minora
labioscrotal swellings: scrotum; labia majora
male external genitalia development
urethra composed of both endoderm (urogenital sinus) and invaginated ectoderm. testes begin dropping. fusing of the urogenital folds to form body of penis. erectile tissues (surface ectoderm) and urethra. further invagination around glans = foreskin.
female external genitalia development
regression of genital tubercle, expansion of labioscrotal swellings
role of gubernaculum
gubernaculum tugs down on gonads in males and females (but more in males) through passage called inguinal canal between abdominal wall. there is a hormone that determines how much the gubernaculum tugs the gonads down. males have a passage from the scrotum thru the abdominal cavity.
gubernaculum in women actually sticks around and becomes part of the ovary after pulling gonads down into the pelvis.
alveoli/air sacs in lungs covered in capillaries — connection between us and atmosphere
relatively high CO2 and low O2 in capillaries leading to the alveoli; the capillaries coming away from the alveoli are high O2 and low CO2
passive diffusion of O2 and CO2 across the membrane in either direction
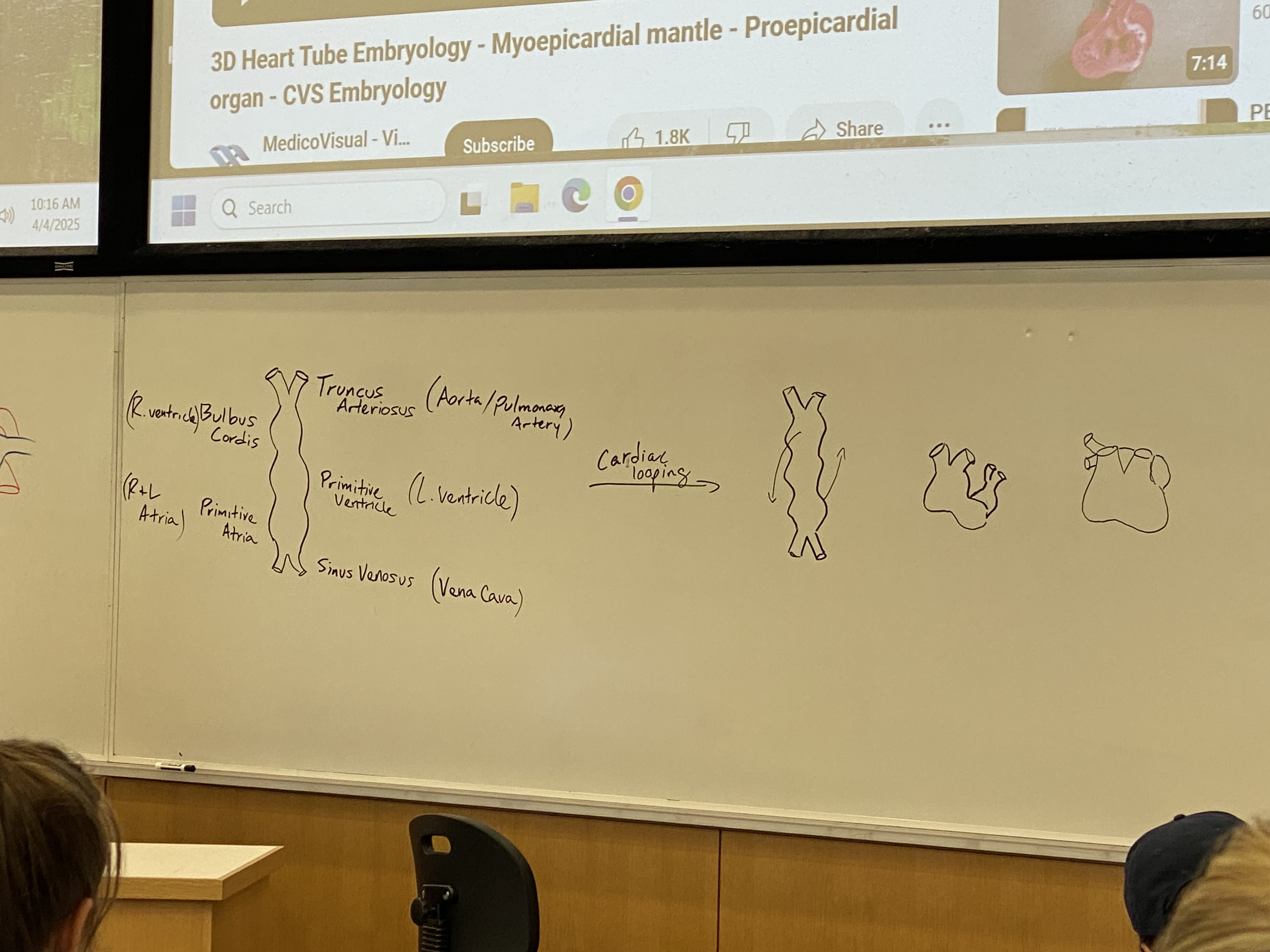
describe cardiac looping
top part w ventricles starts looping to (its right, my left) coming out at me + down
atria part starts doing the opposite, looping to (its left, my right) going back into the board + up
process continues until primitive atria loops behind, up, and back to rest on top of the primitive ventricle + bulbus cordis
all of the chambers will fuse again so we will have one big empty heart sac; chambers will have to be built after
two pieces of evidence we evolved from fish
gas bladder → lungs
pharyngeal arches — in fish, those were the circulation to each of the individual gills. in our development, they eventually differentiate into parts of neck, head, inner ear, etc
adult circulation
have cells dropping off CO2 into blood after cell respiration thru vena cavae into right atrium to r ventricle to pulmonary artery to lungs. CO2 dropped off thru passive diffusion; RBCs pick up oxygen. pulmonary veins to l atrium to l ventricle to aorta to rest of body. drops off oxygen and picks up CO2 waste from tissues.
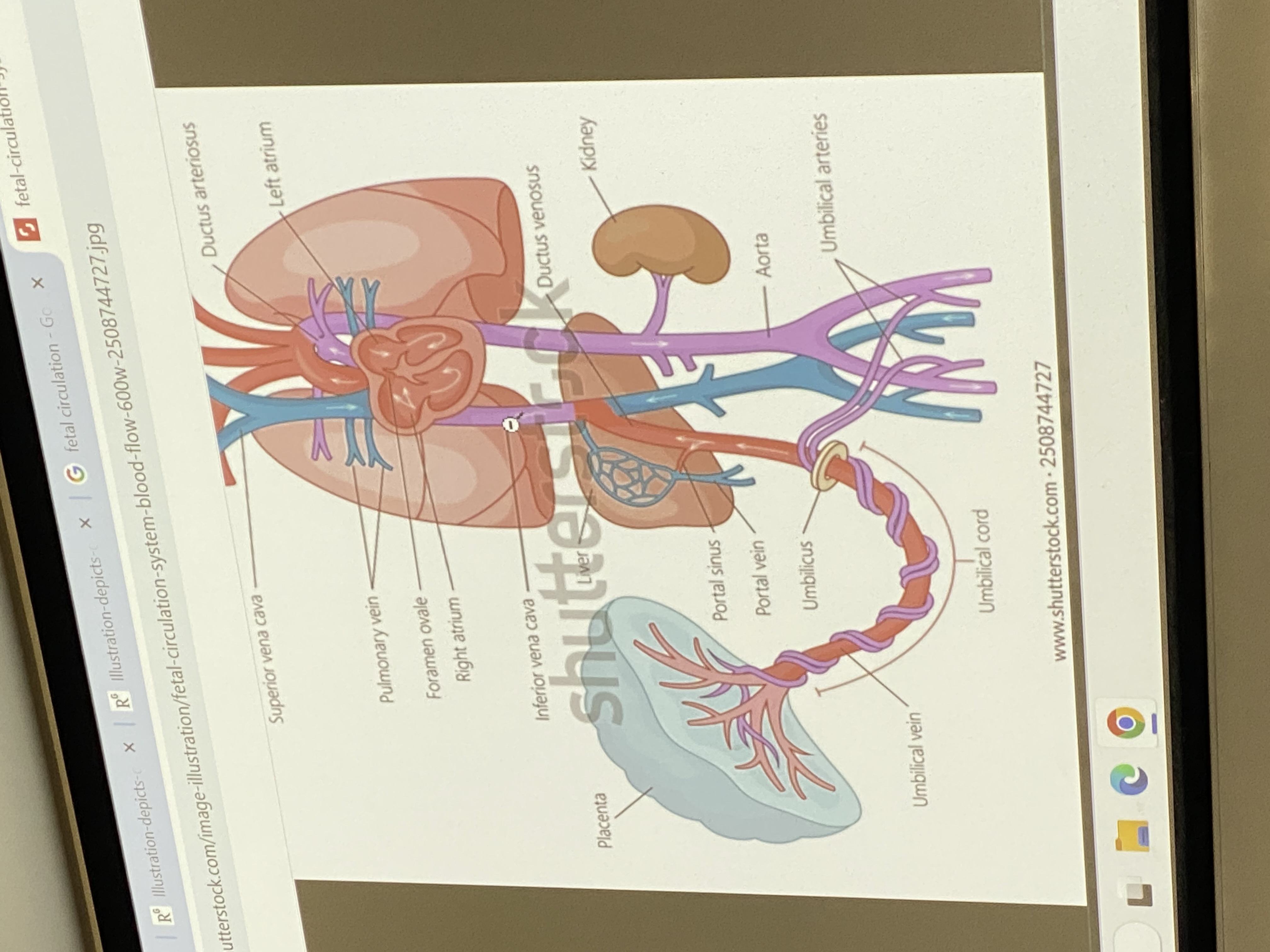
overview of fetal circulation
umbilical vein hooks up to inf vena cava thru liver. semi oxygenated blood from mom heading towards the fetal right ventricle. foramen ovale — right atria pumps mom’s oxygenated blood and some gets squished into the left atrium, into left ventricle, into aorta, out thru systemic circulatory system to aid in growth/dev of fetus. some, as normal, gets pumped from right atria to right ventricle and out to go to lungs — but no gas exchange happening yet ofc.
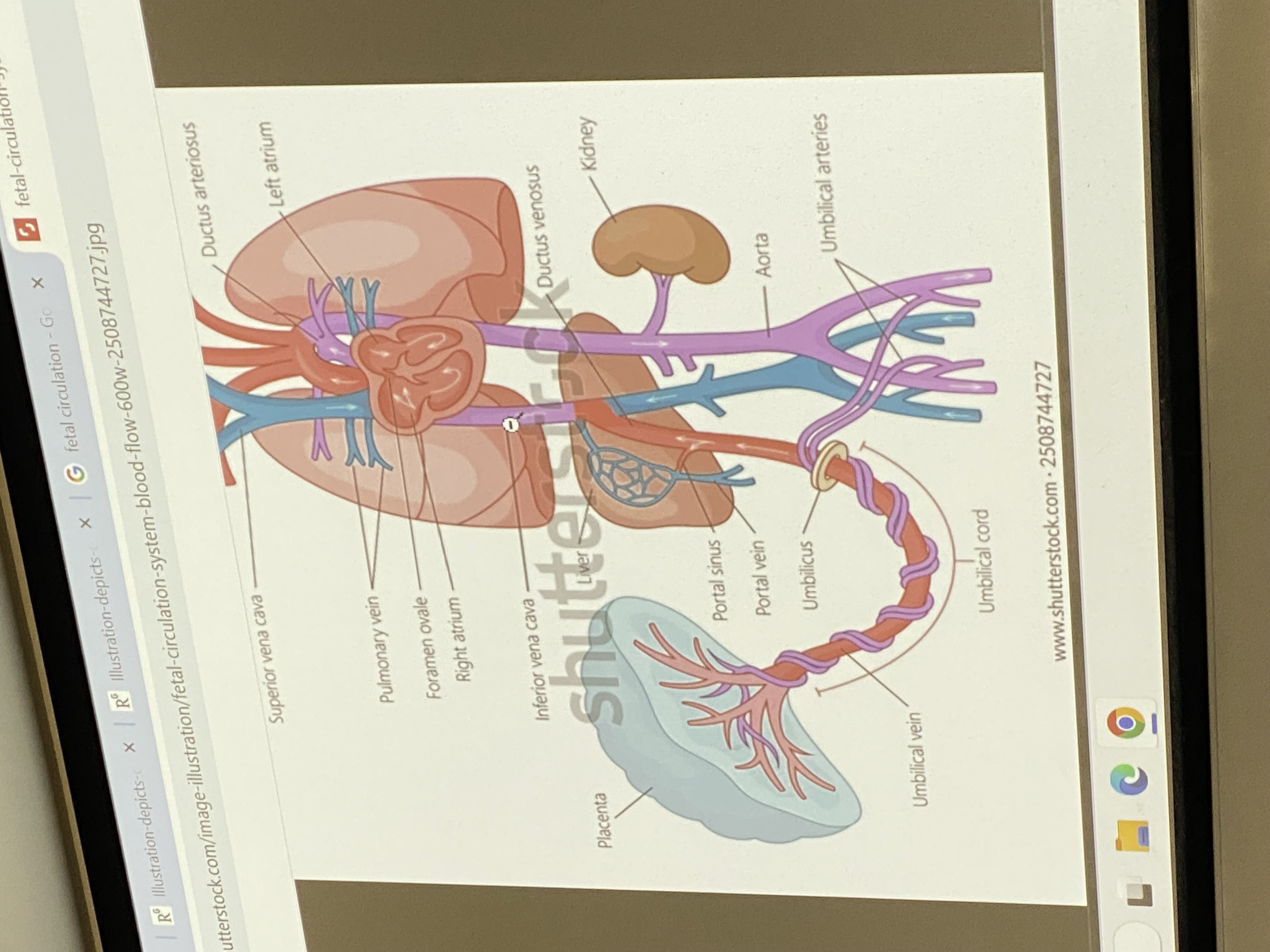
how does heart structure change upon first breath?
the moment infant takes first breath, the pressure in the different parts of the heart changes drastically — septum secundum is forced over the septum primum and closes the foramen ovale + osteum secundum, so they seal off and become atrial septum. extra little channels at birth also degrade — ductus arteriosis closes off and becomes a ligament; extra blood vessels that serve as blood connection to placenta will close off and degrade
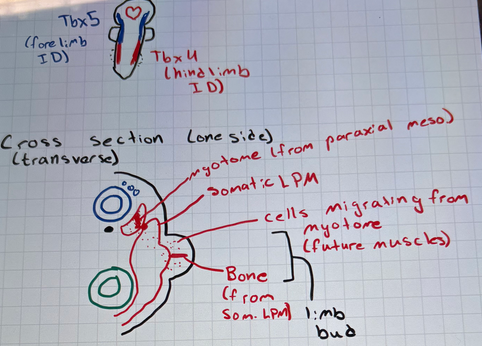
whatever this is. draw it if time ig
.
draw posterior view of limb bud development
purpose of lens and iris in eye dev?
focus the incoming light/image onto retina
analogous to film of camera
draw the transverse section of forebrain after neurulation
neural tube is green
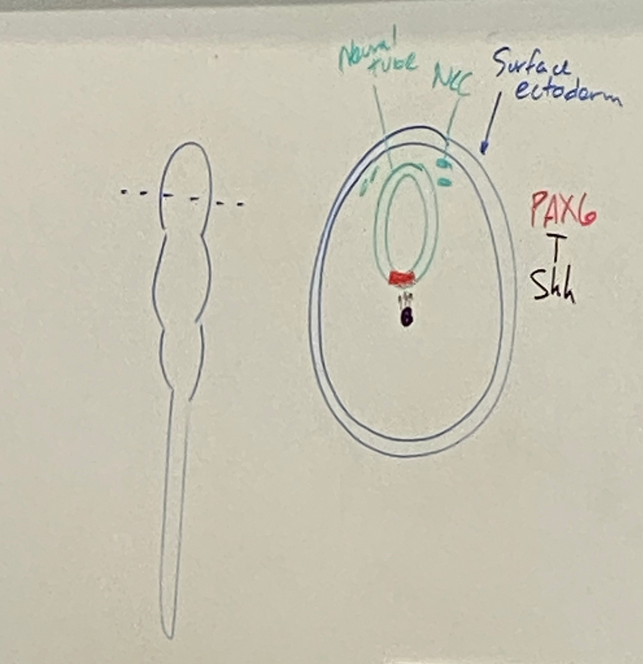
prechordal plate secretes ___? what does it do to the anterior vs posterior ends of embryo?
secretes morphogens. causes anteriorization — anterior cells have certain receptors so they are competent and can receive the signal. posterior cells are not competent and will not receive the signal
what is Shh responsible for in eye field development?
turn off pax6
lead to a LOT of cell division in neural tube ventrally → pax6 expression on the borders (where Shh is not active) turns into two eye fields moving apart from each other
also causes two projections coming off of neural tube in the forebrain where pax6 is expressed
respiration equation
which is reduced?
C6H12O6 + O2 → CO2 + H2O + energy
O2 is reduced to H2O
what happens if O2 is not completely reduced to H2O?
incomplete reduction → reactive oxygen species (ROS)
aging = ?
cellular damage and DNA damage
what two things does foxO do?
DNA repair and ROS protection
provide a developmental/evolutionary explanation for why, as a tetrapod, swallowing can be dangerous
lungs evolved from fish’s gas bladder, which controls buoyancy and was an outpocket of the digestive system. became more and more convoluted and associated w cardiovascular system and ultimately became the lungs
what would you expect to happen in an embryo to which Shh was over-expressed at the ventral midline of the mammalian face/forebrain?
two eye fields over-separate and cause the eyes to develop further apart due to over-proliferation of cells in the midline. if this hypertelorism is particularly severe, eyelessness occurrs like in the blind cave fish.
Limb patterning shares conspicuous similarities with A-P patterning in vertebrates. What are these similarities and what might this inform us about the evolution of development, in general?
Limb patterning is very similar to A-P patterning in vertebrates for many reasons, but one of the most notable similarities is the role of Hox genes in segmentation along an axis, though in limb patterning it is along the proximal-distal axis rather than the anterior-posterior axis. They even both follow the rule of collinearity, meaning that the order of patterning and segmentation corresponds to the order of the genes along the chromosome.
Similarities such as these suggest that these patterning mechanisms were actually just the same process co-opted evolutionarily for the development of different structures and patterning. In other words, the patterning development processes did not each independently evolve; rather, any relevant differences appeared later in the evolutionary history. This somewhat resembles one of the trends seen in biochemistry, as new pathways of development and chemical pathways do not each gradually appear from nothing over many generations. Instead, it is more efficient to re-use and co-opt the same pathways that are already used by other processes, even if they do not initially seem similar enough to be able to do so.
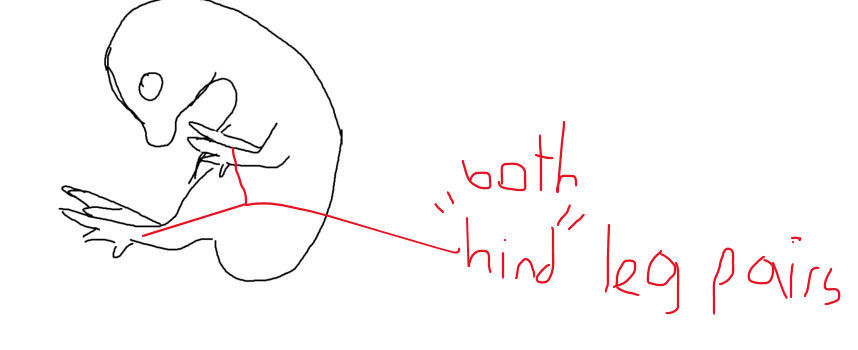
Tbx4 and Pitx1 are key transcription factors that specify limb identity. Draw a picture of what would happen to a late-stage chicken embryo if these two genes were ectopically activated in the forelimb field.
Tbx4 and Pitx1 are both responsible for hindlimb identity. If they were to be expressed in the forelimb field, then the chicken embryo would develop leg-like structures in what would normally be the wings. For example, the digits would appear more like toes and the wings would have joints and muscles that more closely resemble the legs than a typical wing.
in limb development, what happens if a hox gene is missing?
the entire corresponding segment is just lost. behaves more like a gap gene than a hox gene mutation lol
describe the process of cardiac progenitors → first heart tube/trilaminar disc → shorter, thicker heart tube
all driven by Nkx2.5
gastrulation occurs and the cardiac progenitors dive into primitive streak and arrange themselves past anterior to oropharyngeal membrane. first as a cluster of cells known as the cardiac crescent, then reorganize to form first heart tube. at this point, are nonfunctional. this is the triliaminar disc. lateral folding and cranial-caudal folding of the trilaminar disc. bring the parts together and fuse to create a shorter, thicker primitive heart tube that will hook up to circulatory system and become functional.
describe the sequestration and migration of germ cells
germ cells deposit/sequester themselves in yolk sac and/or hindgut to hide from all the signalling going on. start crawling thru digestive system, go across dorsal mesentery, deposit selves in genital/gonadal ridge until they get the proper signals to tell them what to differentiate into.