Organic Chemistry 2510 Midterm 2 Osu
1/184
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
185 Terms
The bond strength of C-X decreases as
size of X increases
F>Cl>Br>I
bond dissociation
The bond between carbon and halogen is made up of an
sp3 orbital on carbon and a p orbital of the halogen
Short bonds are stronger than
longer bonds
Boiling points of haloalkanes are generally higher than
corresponding alkanes; due to dipole dipole interactions
Boiling points also rise with increasing
size of the halogen
polarizability
degree to which the electron cloud is able to deform
nucleophile
substances that contain an unshared electron pair
Nucleophilic substitution
reagent attacks the haloalkane and replaces the halide
Negatively charged nucleophile reacts with a haloalkane to yield
a neutral substitution product
An uncharged nucleophile yields
a positively charged substitution product( with the counteranion it becomes a salt)
substrate
starting organic material
Bimolecular Nucleophilic Subsitution
two reactants interact in one step
nucleophile attacks substrate with simultaneous expulsion of the leaving group
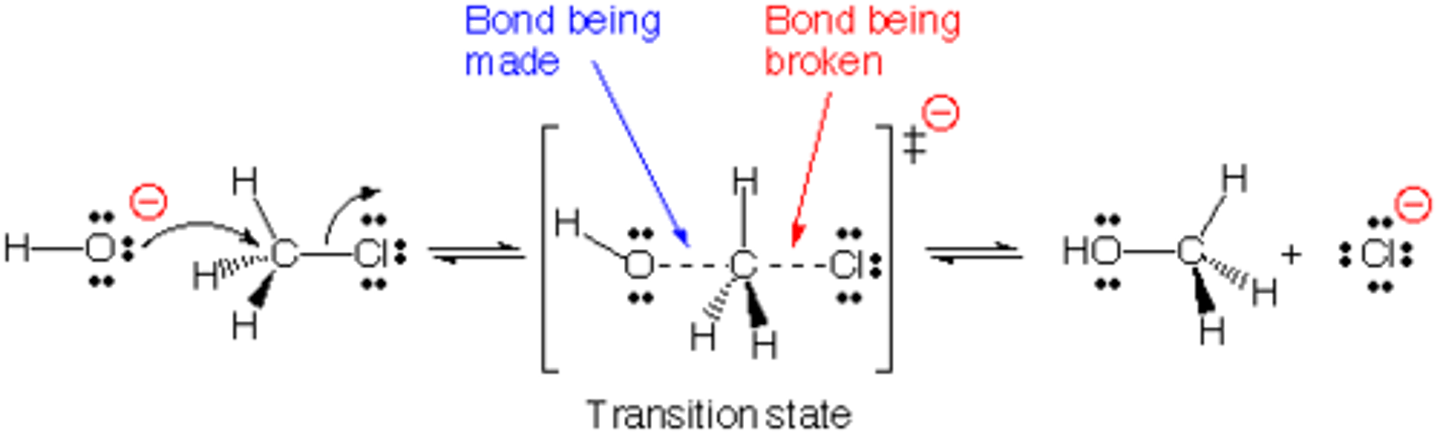
Sn2 is a
concerted reaction
backside displacement
the nucleophile approaches the carbon from the side opposite the leaving group
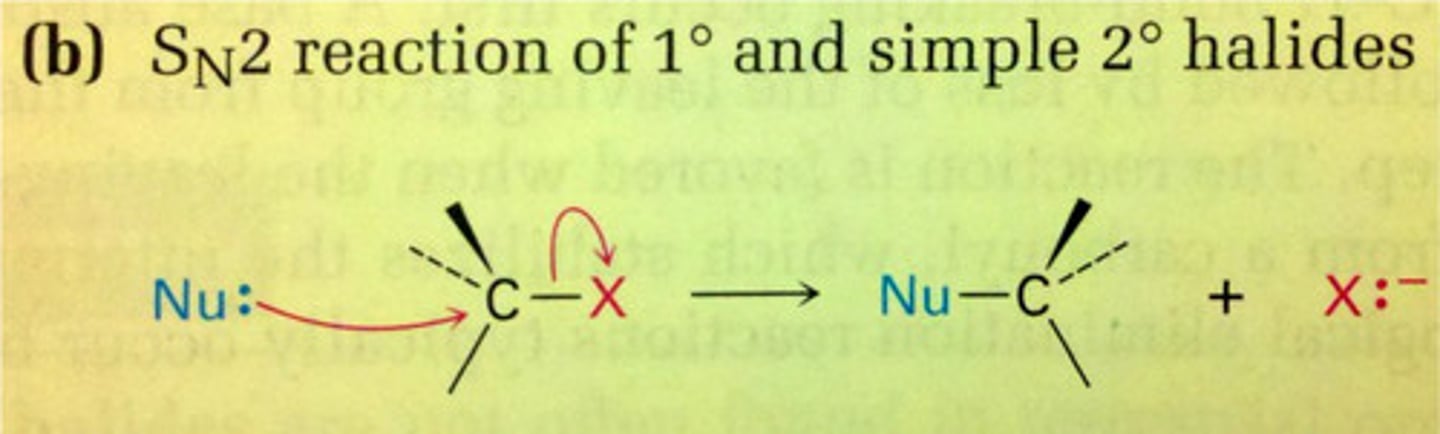
Inversion of Configuration
occurs with all Sn2 reactions
S goes to R, R goes to S
Stereospecific
a process whose mechanism requires that each stereoisomer of the starting material transform into a specific stereoisomer
As the nucleophile approaches the back lobe of the sp3 hybrid orbital used by the carbon to bind to the halogen, the rest of the molecule becomes
planar at the transition state by changing the hybridization to sp2.
Double inversion sequence of two Sn2 processes gives
desired product(if you want to maintain the same configuration); retention of configuration
Facility of Sn2 reactions depend on several factors
-nature of leaving group
-reactivity of nucleophile
-and the structure of the alkyl portion of the substrate
Leaving group ability is correlated with
its capacity to accommodate a negative charge
For halogens, leaving group ability increase
down the column
Sulfur Derivatives are also good
leaving groups
ROSO3- and RSO3-
Leaving group ability is inversely related to
base strength
Weak bases are the best
to accommodate negative charge and are the best leaving groups
Good leaving groups are the
conjugate bases of strong acids
Nucleophilicity depends on
charge,basicity,solvent,polarizability, and the nature of the substituents
increasing negative charge increases
nucleophilicity
Nucleophilicity decreases
to the right of the period table
The more basic the nucleophile
the more reactive
Increasing negative charge has a greater effect
than moving right to left on the periodic table for nucleophilicity
Nucleophilicity increases down
down a column
Solvents capable of hydrogen bonding are called
protic
Switching a protic solvent with an aprotic solvent
-reactivity of the nucleophile is raised
-base strength overrides solvation, thus F is better than I
sterically hindered nucleophiles are
poorer reagents
DMF is
aprotic
Branching at the reactive carbon
decreases the rate of the Sn2 reaction
Relative Sn2 displacement reactivity
methyl>primary>secondary>>tertiary
Branching on B-Carbon
retards subsititution
solvolysis
a substrate undergoes substitution by solvent molecules
For hydrolysis, the rates of reactions
increases with the substitution on the reacting carbon, thus tertiary>secondary>primary
Overview of Sn2 reaction
-has second order kinetics
-generates products stereospecifically with inversion of configuration
-Is the fastest with halomethanes and slower with primary and secondary halides
-takes place very slowly with tertiary substrates if at all
Overview of Solvolyses or Sn1
-follow first order rate law
-are not stereo-specific
-characterized by opposite order of reactivity
Uni molecular substitution
Sn1, only one molecule participates in the rate determining step
-the rate does not depend on the concentration of the nucleophile
Sn1 consists of three steps
1)formation of carbocation
2)attack by nucleophile
3)deprotonation
For solvolysis, a large excess of nucleophilic solvent ensures
complete solvolysis
To minimize electron repulsion, the positively charges carbon assumes
trigonal planar geometry,sp2 hybridization
Sn1 reactions obtain
racemic products
Sn1 rate increases as solvent
polarity increases
Protic solvents accelerate Sn1 because
it stabilizes the transition state by hydrogen bonding with the leaving group
Sn2 reactions are accelerated in
aprotic solvents
Sn1 speeds up with
better leaving group
Sn1 is not effected by the strength
of the nucleophile, but strengths may affect product distribution
Primary haloalkanes undergo only
bimolecular substitution
Relative stability of carbocations
primary
What is the reason for carbocation stability
hyperconjugation
The pathway of secondary haloalkanes depends on
the solvent, leaving group, and nucleophile
What makes Sn1 favorable for secondary substrates
substrate bearing good leaving group, poor nucleophile, and polar solvent
What makes Sn2 favorable for secondary substrates
high amounts of good nucleophile, reasonable leaving group, and aprotic solvents
Sn2 compared to Sn1 is
greener
Elimination
removal HX with the simultaneous generation of a double bond
E1
uni molecular eliminations
rate determining step is the formation of the carbocation
which hydrogens can participate in E1
any hydrogen posititions on any carbon next to the center bearing the leaving group
E2
bimolecular elimination
rate of alkene formation proportional to the concentrations of both the halide and the base
E2 reactions proceed in
a single step
three changes take place in E2 reactions
1)deprotonation by the base
2) departure of the leaving group
3) Rehybridization of the reacting carbon ceneter from Sp3 to Sp2 to furnish a double bond
How E2 and E1 differ
E2- base does not wait for carbocation formation because it is more aggressive
E1-Carbocation first, then base is protonated
If the leaving group is equatorial
all the corresponding hydrogons are not axial, making the elimination process slower
anti transition state is preferred
so the the base can extract a hydrogen the same time the leaving group leaves
Weakly basic nucleophiles give
substitution
Sn2-primary Secondary
Sn1-Tertiary
Weak nucleophiles such as water and alcohols react
at decent rates only with secondary and tertiary halides, substrates capable of Sn1, elimination is minor
Strongly basic nucleophiles give more
elimination as steric bulk increases
As steric bulk increases around carbon bearing leaving group,
substitution is retarded relative to elimination because an attack on carbon is subject to more steric hinderance relative to a attack on hydrogen
Branched primary substrates give
equal amounts of Sn2 and E2
Steric Bulk on the nucleophile
hinders attach at the electrophilic carbon, making elimination predominate
Good nucleophile weak base
subsitution more likely
Good nucleophile strong base
elimination increases
Sterically unhindered primary haloalkanes
substitution
Sterically hindered primary, secondary, and tertiary haloalkanes
elimination
Sterically unhindered nucleophiles that are strong bases
substitution
Sterically hindered nucleophiles that are strong bases
elimination
Primary Haloalkanes reactivity summary
-unhindered primary alkyl substrates will always yield Sn2 products except with sterically hindered nucleophiles then E2 becomes predominant
-However good nucleophiles will furnish Sn2
-Strong bases will give E2
-poor nucleophiles give NR
Secondary Haloalkanes reactivity summary
-Good nucleophiles favor Sn2
- Strong bases favor E2
-weakly nucleophilic polar media give E1 Sn1
Tertiary Reactivity haloalkanes summary
-strong bases(E2)
-non basic media gives E1 and Sn1
The name of the alcohol is based on the chain
containing the OH substiuent
When there is more than one hydroxyl group along the alkane stem
name is followed by diol, triol, etc
Alcohol boiling points are much higher than
their corresponding alkanes and haloalkanes
The high boiling points of alcohol are a result of
hydrogen bonding
Alkanes are
hydrophobic
Alcohols solubility in water
good; hydrophilic
The larger the alkyl part of an alcohol the
lower its solubility in water
The oxygen in alcohols hybridization is
sp3
Deprotonation of alcohols give
alkoxides
Protonation of alcohols gives
alkyloxonium ions
Why alcohols are acidic
the electronegativity of the O stabilizes the alkoxide molecule
Effects of branching on Alcohol acidity
methanol> primary>secondary>tertiary
Presence of halogens
increases acidity
Inductive effects
transmission of charge through sigma bonds in a chain of atoms; stabilizes the negative charge of the alkoxide Oxygen
Amphoteric
may be a base and acid
Synthesis gas
pressurized mixture of CO and H2 to make Methanol
usually consists of catalyst consisting of copper, zinc oxide, and chromium(III) oxide
changing catalyst to Rhodium leads to 1,2ethanediol(antifreeze)
Ethanol is prepared in large quantities by
fermentation of sugars or phosphoric acid catalyzed hydration of ethene