NMR spectroscopy
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
what information can we get from an NMR spectrum
How many types of H’s in a compound (number of unique signals)
What types of H are in a compound (chemical shift of the signals)
How many of each type of H are in the compound (integration of signals)
How many H’s are “next door” (signal splitting from spin-spin coupling)
how do you determine how many types of H’s are in a compound from the spectrum
the number of unique signals
what do we mean when we say “types” of H’s?
if they’re different in any way, they’re different types. for example, in a CH3, all 3 hydrogens are the same (because of free rotation). however, in R-CH2-CH3, there are two different types— one is part of a CH3, one is part of CH2. also matters what its central carbon is attached to— i.e. CH3-O-CH2-CH3 has three different types of hydrogens
how do we determine what types of H’s are in a compound?
chemical shift of the signals
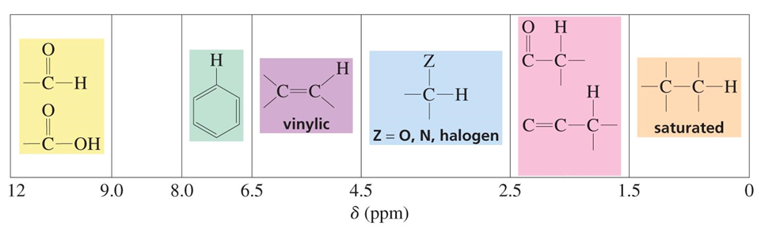
chemical shift?
chemical shift of 9.0-12.0
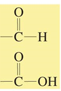
chemical shift?
chemical shift of 6.5-8.0
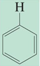
chemical shift?
chemical shift of 4.5-6.5
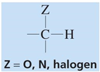
chemical shift?
chemical shift of 2.5-4.5
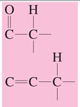
chemical shift?
chemical shift of 1.5-2.5
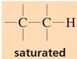
chemical shift?
chemical shift of 0-1.5
which side of a spectrum is upfield vs. downfield?
downfield is towards left (higher numbers); upfield is towards right (lower numbers).
how is the height of each signal relevant?
the area under each signal is proportional to the number of H’s causing that signal — integrating the signals will thus give a ratio indicating the # of H’s of each type. make sure when doing this that you get (mostly) whole numbers. you can’t have ½ a hydrogen, but 0.95 is probably close enough to consider as 1, for example.
after integrating a signal, you get a ratio of 1.5 : 1. is this a reasonable/valid ratio of the H types? if not, what should you do?
no— multiply by integers until you get the lowest ratio of whole numbers, and that will most likely be the exact number of hydrogens of each type in the actual molecule. in this example, multiplying by 2 gives us 3:2 — reasonable! but 6:4 is also reasonable, so to determine if it’s actually correct, you can use the molecular weight (if you know it) to calculate if your ratio is right.
how to calculate ratio of H types in a compound based on the spectrum?
label signals a, b, etc starting with the furthest upfield as a. find integration of each signal (done by computer; will be given to us). divide each one by the lowest number. if the numbers aren’t close enough to being whole, multiply by integers. use MW info (if available) to check that your ratio is accurate. often, the ratio will be the lowest ratio of whole numbers.
how do you determine how many H’s are “nearby”?
signal splitting from spin-spin coupling — when a different type of hydrogen is within 3 sigma bonds away (usually on a next door carbon, but they may be on the same carbon in some cases). if N= the number of that different type of H’s nearby, then the signal is split into N+1 peaks. note that if there’s two different types of H’s nearby, they will split the signal independently — leads to doublet of doublets, triplet of doublets, etc. note the difference between a doublet of doublets vs a quartet, for example
do identical hydrogens split each other?
no— only different types, 3 sigma bonds away or less.
the number of peaks in a signal is called what? how do you name them?
multiplicity — singlet, doublet, triplet, quartet, etc.
a signal split more than once might show up as a “multiplet” — undefined number of peaks
how do you tell the difference between a doublet of doublets and a quartet, since both have 4 peaks?
a doublet of doublets’ peaks will all be roughly the same size, because a doublet will have a 50/50 ratio of each spin, and this is a doublet OF a doublet — so they’re all equal ratio.
a quartet’s inner two peaks will be taller than the outer two. check PPT slide 23 to see a breakdown of signal splitting in a triplet and why the middle peaks are taller.
are split signals usually symmetric?
no — one side is often higher than the other. this is known as leaning— coupled signals will “lean” towards each other! → can be a hint for which signals are splitting each other on a spectrum. note that doublets typically are symmetric though, due to their spin ratio being 50/50.
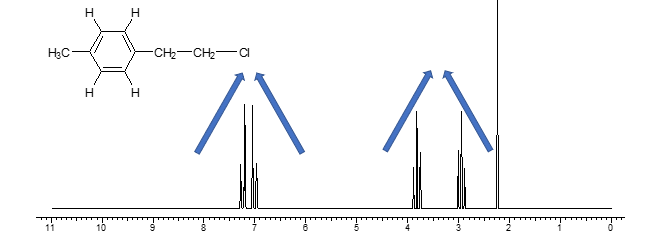
are things affecting chemical shift additive?
yes — see PPT slides 29 and 30
what is the coupling constant (J) and why is it relevant? (how can you use it?)
the distance in Hz between two adjacent peaks of a split NMR signal. J=Δδ(ppm)×operating frequency of the instrument. probably won’t be asked to calculate, and won’t be able to measure using a ruler because it’s so tiny and specific.
within a single signal, all peaks will be equally spaced (J), and coupled signals (from H’s that split each other) will also have the same J. this allows us to identify which signals are splitting each other. J values can also help identify the spatial relationship of coupled H’s. keep in mind that since we won’t calculate/measure J ourselves, we can’t really use any this information unless the J values are given to us, or if the peaks are noticeably REALLY far apart.
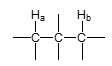
J value?
J = 0 Hz
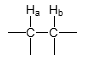
J value — vicinal alkyl group?
J = 7 Hz
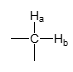
J value — geminal alkyl group?
J = 12 Hz
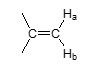
J value — geminal vinyl group?
J = 2 Hz
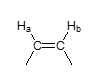
J value — vicinal cis vinyl group?
J = 10 Hz
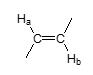
J value — vicinal trans vinyl group?
J = 15 Hz
how do protons on O and N behave on a 1H NMR?
integration can be higher or lower than expected (often a result of rapid proton exchange, esp with H2O)
will sometimes participate in splitting, sometimes not (also a result of rapid proton exchange)
often have variable chemical shift and can really show up anywhere on the spectrum (often a result of intermolecular H bonding)
often give broad signals (also due to intermolecular H bonding)
steps/strategy to interpreting, given MW, IR spectrum, and the NMR spectrum of a molecule
if not given MW, find it using mass spec.
use IR for parts list, only including things you’re certain of.
use NMR integrations to determine actual # of Hs in the molecule
use the actual #s of Hs to determine certain parts — i.e. a signal representing 3 Hs is pretty much guaranteed to be a CH3; a signal for 2 Hs is typically either CH2 or part of an aromatic ring (use chemical shift + MW to determine)
add up the MW of everything in your parts list and determine how far off you are from the given
ONLY NOW can you start putting things together. use multiplicities + splitting.
look at chemical shift to determine what would drag certain parts downfield
keep in mind how many attachments each part can make — which ones are end pieces vs. middle pieces?
info provided by 13C NMR spectroscopy
how many types of C are in a compound (# of unique signals)
what types of C are in a compound (chemical shift)
can you use integration information on a 13C NMR?
no — integration is not proportional to # of carbons
is there any splitting with 13C NMR?
no — it’s run in such a way that the Hs are decoupled from the Cs so that you will only see singlets. otherwise, it would be too messy to see
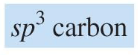
chemical shift?
chemical shift of 0-50
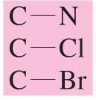
chemical shift?
chemical shift of ~50
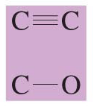
chemical shift?
chemical shift of 50-100
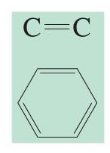
chemical shift?
chemical shift of 100-150
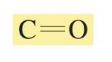
chemical shift?
chemical shift of 150-200
in a 13C NMR, what region is especially important?
the carbonyl region (150-200) is especially important. aldehydes and ketones will be further downfield than “carboxylic acids and carboxylic acid derivatives” in which the C=O is attached to a hetero atom instead of C or H