SECTION 05: ENZYME INHIBITION
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
Q: Why does the cell need to regulate enzyme activity?
A: To meet metabolic needs, prevent overproduction of products, and avoid shortages of essential molecules.
Q: How is enzyme activity regulated in the cell?
A: Through enzyme regulation methods and environmental/physical factors.
Q: What type of inhibitors are the main focus of this section?
A: Reversible inhibitors mainly and a little bit of irreversible inhibitors (briefly)
What are reversible enzyme inhibitors?
Reversible inhibitors temporarily reduce enzyme activity without permanently damaging the enzyme.
What are the 3 main types of reversible inhibitors?
Competitive
Uncompetitive
Non-competitive
Competitive Inhibitor (CI)
Binds at the active site
Competes with the substrate
Km ↑ (substrate needs to outcompete inhibitor)
Vmax stays the same
Trick: "Competitive = Km increases"
Lineweaver-Burk: Lines intersect on y-axis
Non-competitive Inhibitor (NCI)
Binds to free enzyme and ES complex (not at active site)
Vmax ↓
Km unchanged
Trick: “Non-comp = No change in Km”
Lineweaver-Burk: Lines cross at x-axis
What is the purpose of enzyme inhibitors?
They regulate enzyme activity to maintain balance—preventing too much or too little of a product from forming.
Types of Reversible Inhibitors (4)
Competitive
Uncompetitive
Noncompetitive
Mixed
What is Ki and Ki′?
Ki = affinity of inhibitor for the free enzyme
Ki′ = affinity of inhibitor for the enzyme-substrate (ES) complex
→ Smaller values = stronger inhibition
What is IC₅₀?
The drug concentration that reduces enzyme activity by 50% in vitro.
When are substrate-velocity curves used?
To compare how enzyme speed changes across different inhibitor concentrations—one curve must be without an inhibitor (control).
How does inhibition change the Michaelis-Menten equation?
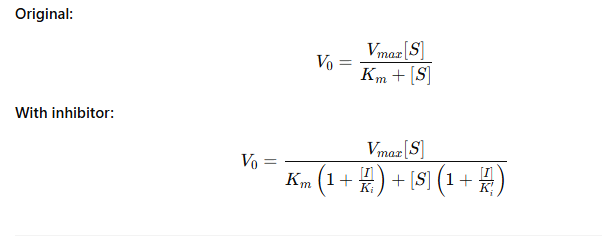
What do the new terms mean?
[I] = Inhibitor concentration
Ki = Inhibitor’s affinity for free enzyme
Ki′ = Inhibitor’s affinity for enzyme-substrate complex
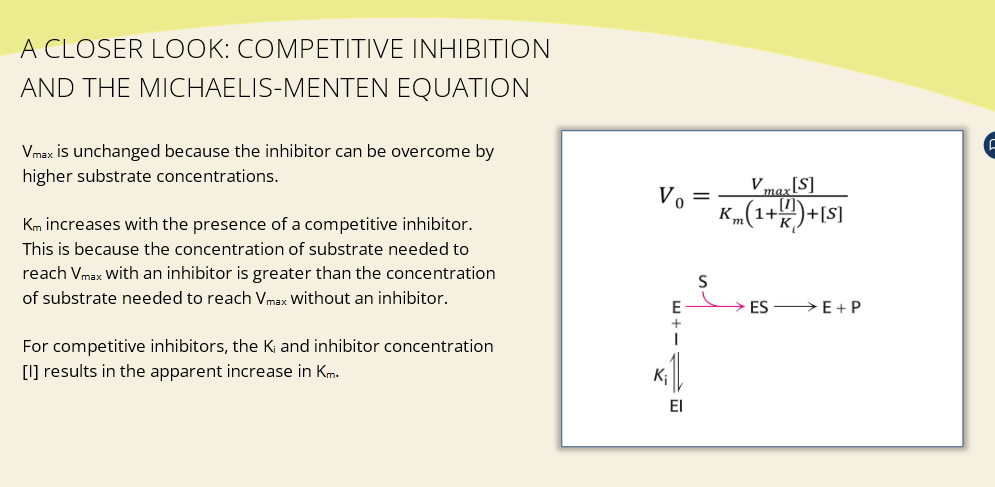
What’s the meaning of the equation change?
If [I] increases, the denominator gets bigger
This slows the reaction rate (V₀)
Whether Km or Vmax change depends on inhibitor type (covered in upcoming slides)
What does the left image show?
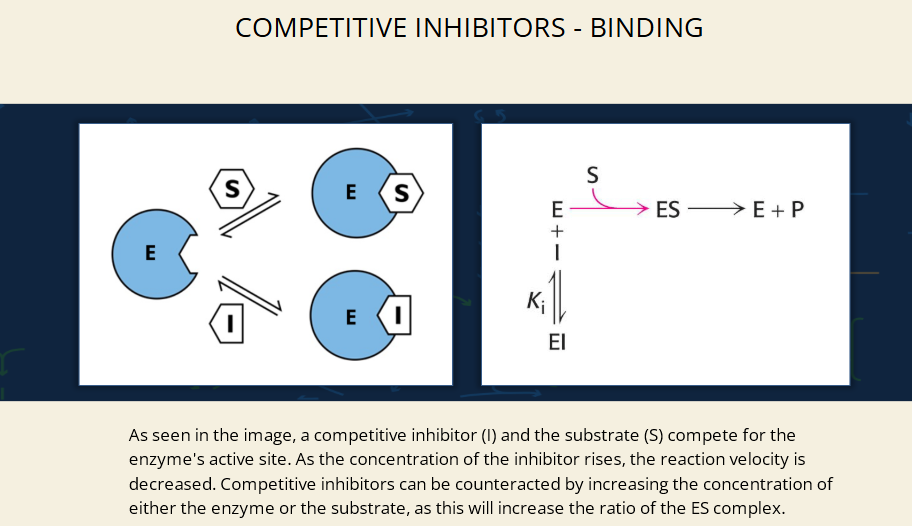
A competitive inhibitor (I) and a substrate (S) are both trying to bind to the enzyme’s active site (E).
If S binds → ES complex → product
If I binds → blocks substrate → no reaction
What does the right diagram represent?
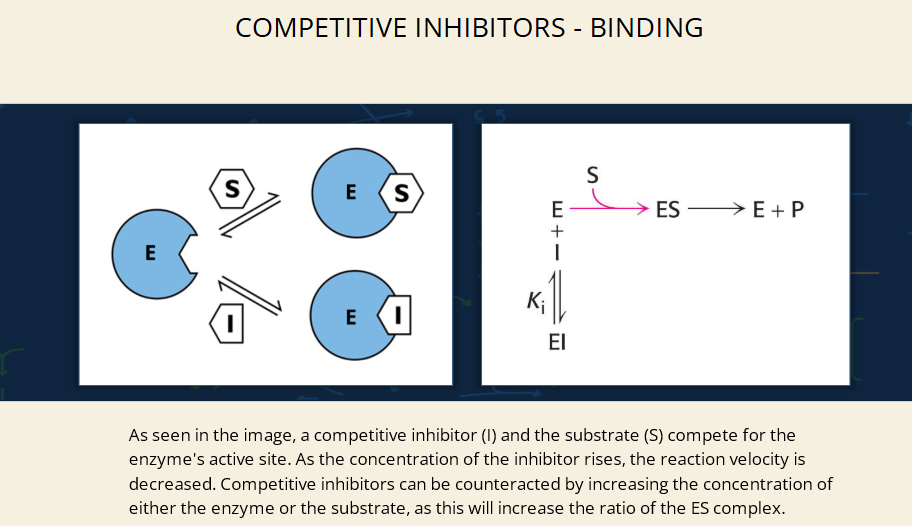
It’s a visual of the reaction:
E + S ⇌ ES → E + P
↕
I ⇌ EI
Where Ki is the binding affinity of the inhibitor.
Can the inhibition be reversed?
✅ Yes!
Adding more substrate (S) outcompetes the inhibitor → reaction resumes.
How do you increase ES complex formation?
By increasing either:
The substrate
The enzyme concentration
This shifts the balance toward product formation.
What do competitive inhibitors do?
Competitive inhibitors compete with the substrate for the enzyme’s active site.
They block the substrate from binding by fitting into the same site.
competitive inhibitors and enzyme kinetics - 📈 Flashcard 2: How do they affect Km and Vmax?
Km increases (you need more substrate to reach half Vmax).
Vmax stays the same (at high substrate concentration, the substrate can outcompete the inhibitor).
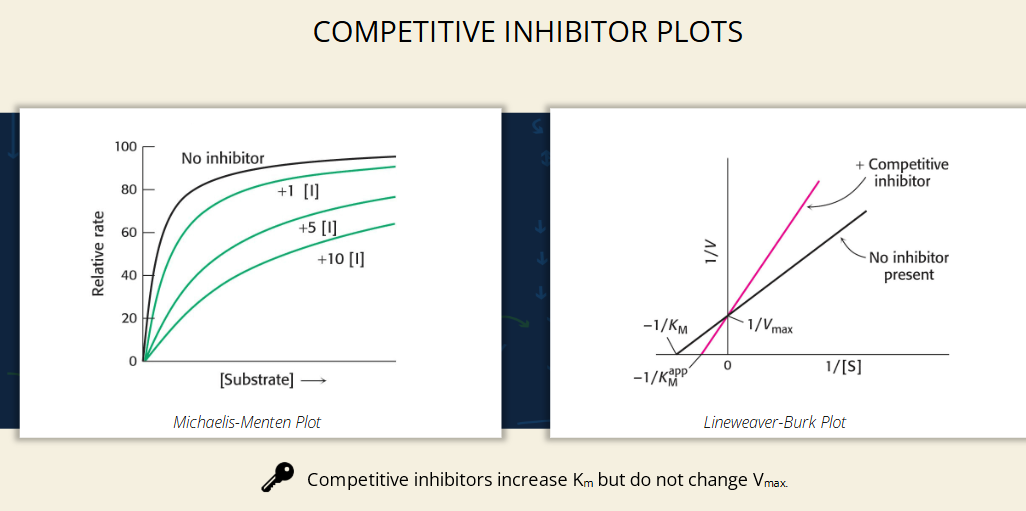
competitive inhibitors and enzyme kinetics - What happens on the Michaelis-Menten plot?
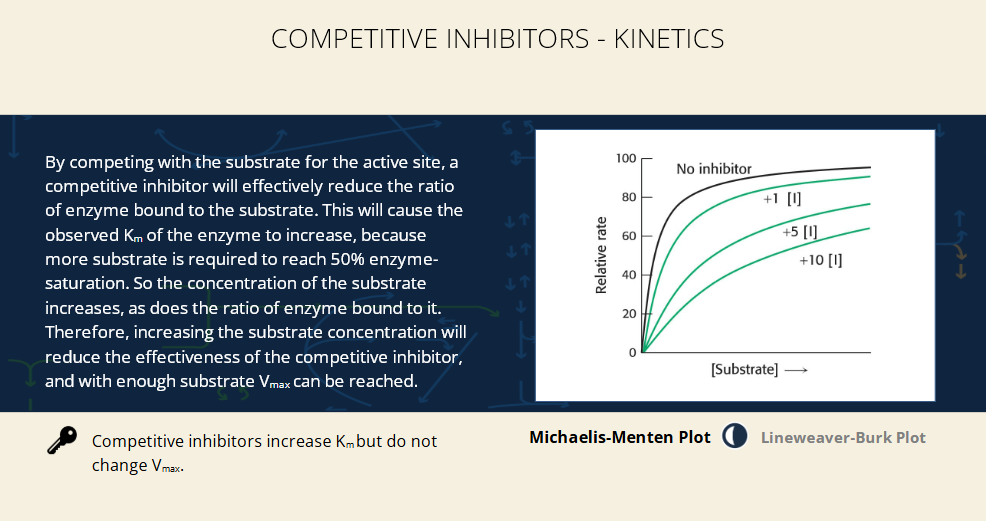
Without inhibitor: Curve reaches Vmax quickly.
With inhibitor: Curve is shifted right (takes more [S] to reach the same velocity).
The top (Vmax) is the same across curves, but they rise more slowly with more inhibitor.
competitive inhibitors and enzyme kinetics - What does the Lineweaver-Burk plot show?
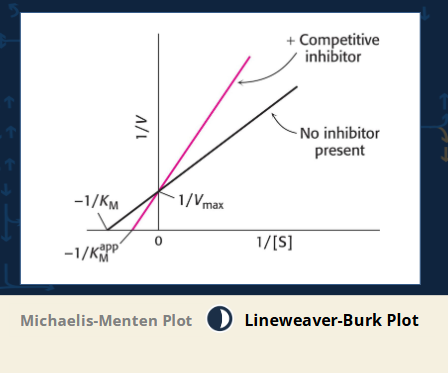
Lines intersect at the Y-axis (Vmax unchanged).
Slope increases with inhibitor = Line gets steeper.
The X-intercept (−1/Km) shifts closer to zero = Higher Km.
Mnemonic: "Competitive = Crosses at Y."
competitive inhibitors and enzyme kinetics - Key takeaway
Competitive inhibitors make it harder for the substrate to bind (↑Km),
but don't change the max speed of the reaction (Vmax stays the same).
What happens to Vmax in competitive inhibition?
Vmax stays the same.
Why? Because if you add enough substrate, it can outcompete the inhibitor for the active site.
competitive inhibition - What happens to Km?
Km increases.
Why? You need more substrate to reach 50% of Vmax when an inhibitor is present.
competitive inhibition - What causes the increase in Km?
The Ki (inhibitor constant) and [I] (inhibitor concentration) together affect the apparent Km.
Formula:
![<ul><li><p>The <strong>Ki (inhibitor constant)</strong> and <strong>[I] (inhibitor concentration)</strong> together affect the apparent Km.</p></li><li><p>Formula:</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/17d6a8eb-1025-48fa-a6c9-7db83c8cacbf.png)
Reaction overview (diagram)
The substrate (S) and inhibitor (I) both compete to bind to the enzyme (E).
The enzyme can either form ES (productive) or EI (blocked).
Only ES → E + P leads to product.
Key takeaway
Competitive inhibitors don’t affect Vmax, but increase Km.
The higher the [I], the more substrate you need to compete.
Activity
📊 Michaelis-Menten Plot

📈 Lineweaver-Burk Plot (Double Reciprocal Plot)
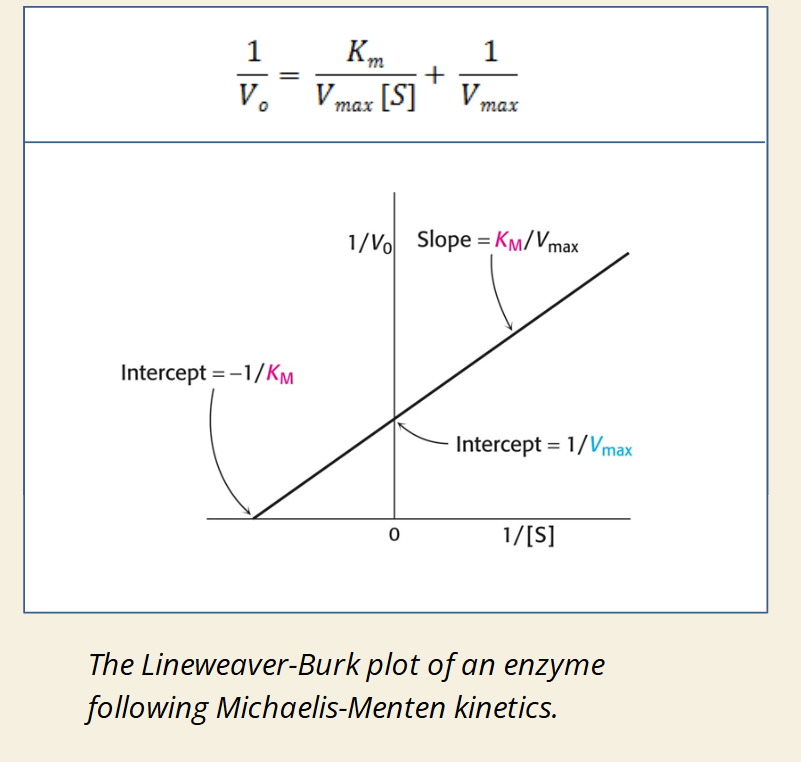
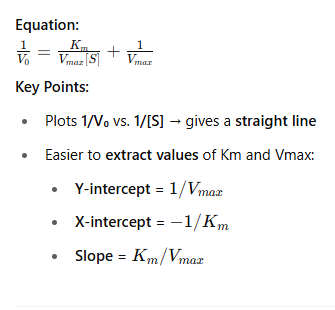
🧠 Use Case:
Michaelis-Menten plot is better for visualizing enzyme behavior.
Lineweaver-Burk plot is better for calculating exact Km and Vmax.
Both help identify effects of enzyme inhibitors (e.g. competitive inhibition increases Km, Lineweaver-Burk shows this as steeper slope).
🧪 Uncompetitive Inhibitors – Binding
Only bind after the substrate is bound (binds to enzyme-substrate complex (ES))
Binds at an allosteric site, not the active site (but often near it)
Binding prevents catalysis → enzyme becomes inactive
Reduces the amount of available active enzyme
Uncompetitive Inhibitors – Binding
When do they bind?
➡ Only bind to the enzyme-substrate complex (ES) (not free enzyme)
Where do they bind?
➡ Bind at a site other than the active site (often near it)
What happens when they bind?
➡ Block enzyme activity – the enzyme can no longer convert substrate to product
➡ Reduces the number of active enzymes available
🔑 Key Idea:
Uncompetitive inhibitors don’t prevent binding of the substrate — they prevent the enzyme from doing its job after binding has already happened.
What are uncompetitive inhibitors?
Q: When do uncompetitive inhibitors bind?
A: Only after the substrate is bound to the enzyme (they bind the enzyme-substrate complex, not the free enzyme).
🟩 Flashcard 2: What happens to Km and Vmax in uncompetitive inhibition?
Q: How does an uncompetitive inhibitor affect Km and Vmax?
A: Both Km decreases and Vmax decreases.
🟩 Flashcard 3: Why does Km decrease?
Q: Why does apparent Km decrease in uncompetitive inhibition?
A: Because the enzyme is "trapped" in the ES complex, which increases its apparent affinity for the substrate.
🟩 Flashcard 4: Why does Vmax decrease?
Q: Why does Vmax decrease in uncompetitive inhibition?
A: The inhibitor prevents the ES complex from forming product, reducing the number of active enzymes.
🟩 Flashcard 5: Michaelis-Menten Plot (left graph)
Q: How does the Michaelis-Menten curve change with uncompetitive inhibition?
A: The curve shifts down (lower Vmax) and left (lower Km). More inhibition = more flattening and left shift.
🟩 Flashcard 6: Lineweaver-Burk Plot (right graph)
Q: What happens to the Lineweaver-Burk plot in uncompetitive inhibition?
A: The lines are parallel (same slope), shifted upward and left (because both 1/Vmax and -1/Km increase in magnitude).
🟩 Flashcard 7: Why are uncompetitive inhibitors more effective at high [S]?
Q: When are uncompetitive inhibitors most effective?
A: When there is lots of substrate, because they need the ES complex to form first.
🟦 Flashcard 1: Binding behavior
Q: Where do uncompetitive inhibitors bind?
A: They only bind to the enzyme-substrate complex (ES), not the free enzyme.
🟦 Flashcard 2: What happens to Km?
Q: What does uncompetitive inhibition do to Km?
A: It decreases Km (makes the enzyme seem to bind substrate better).
🟦 Flashcard 3: What happens to Vmax?
Q: What does uncompetitive inhibition do to Vmax?
A: It decreases Vmax by reducing the number of active enzymes available to make product.
🟦 Flashcard 4: Why is Km lowered?
Q: Why does Km decrease with uncompetitive inhibition?
A: Because the enzyme is trapped in the ES complex, making it appear to have higher substrate affinity.
🟦 Flashcard 5: When is uncompetitive inhibition most effective?
Q: When do uncompetitive inhibitors work best?
A: At high substrate concentrations, since more ES complexes are formed.
uncompetitive - 🟦 Flashcard 6: Equation overview
Q: What’s the uncompetitive Michaelis-Menten equation?
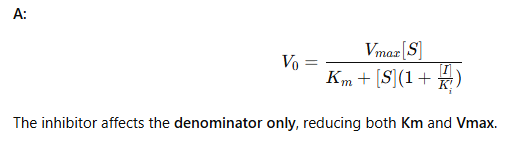
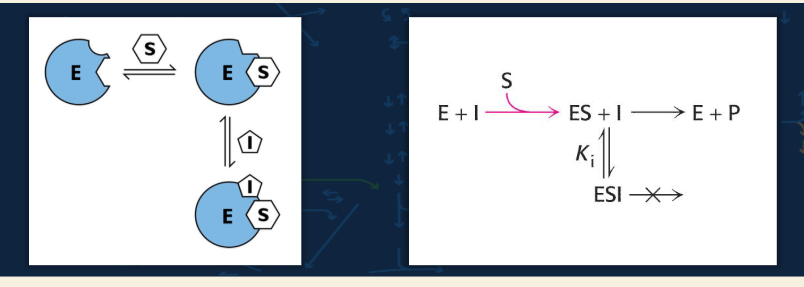
uncompetitive - 🟦 Flashcard 7: Reaction diagram meaning
Q: What does the reaction diagram show?
A:
E + S forms ES
Inhibitor (I) binds only to ES → forms ESI, which is inactive
The enzyme cannot convert substrate to product from ESI
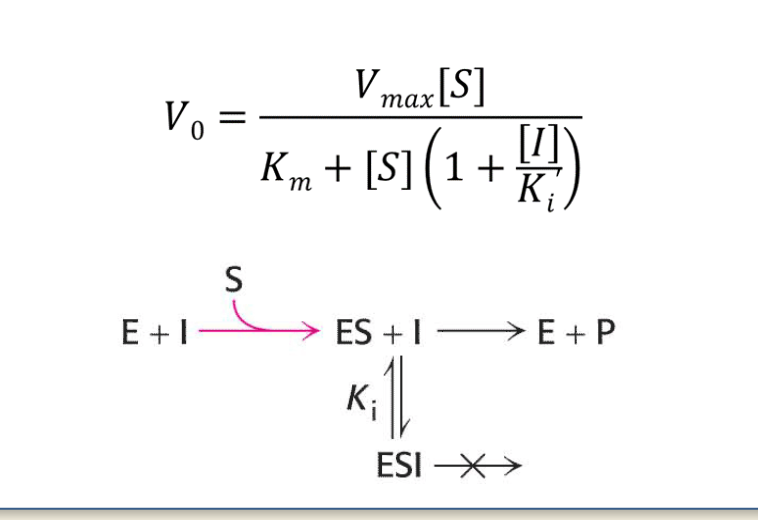
Binding Site
Q: Where do noncompetitive inhibitors bind?
A: To an allosteric site, not the active site.
🟩 Flashcard 2: Binding Target
Q: What can noncompetitive inhibitors bind to?
A: They bind equally well to the free enzyme [E] and the enzyme-substrate complex [ES].
🟩 Flashcard 3: Key Difference
Q: How are noncompetitive inhibitors different from competitive and uncompetitive inhibitors?
A:
Competitive binds only E
Uncompetitive binds only ES
Noncompetitive binds both E and ES
Flashcard 1: Binding Targets
Q: What does a noncompetitive inhibitor bind to?
A: It binds equally well to the free enzyme (E) and the enzyme-substrate complex (ES).
🟪 Flashcard 2: Effect on Enzyme Function
Q: What happens when a noncompetitive inhibitor binds to the enzyme?
A: The enzyme’s ability to bind or release substrate is reduced, making it less catalytically active.
🟪 Flashcard 3: Main Outcome
Q: What is the result of noncompetitive inhibitor binding on enzyme concentration?

A: It lowers the effective enzyme concentration in the reaction—even if some enzyme is present, it's inactive when the inhibitor is bound.
Flashcard 1: What do noncompetitive inhibitors bind to?
🧬 Both the free enzyme (E) and the enzyme-substrate complex (ES)
✅ With equal affinity
🧷 Binding occurs at an allosteric site, not the active site
Flashcard 2: What is the effect of noncompetitive inhibitors on Km and Vmax?
📉 Vmax decreases
➖ Km stays the same
🧠 Because binding doesn't affect substrate binding, just catalytic activity
Flashcard 3: Michaelis-Menten Plot Explanation (Image 2)
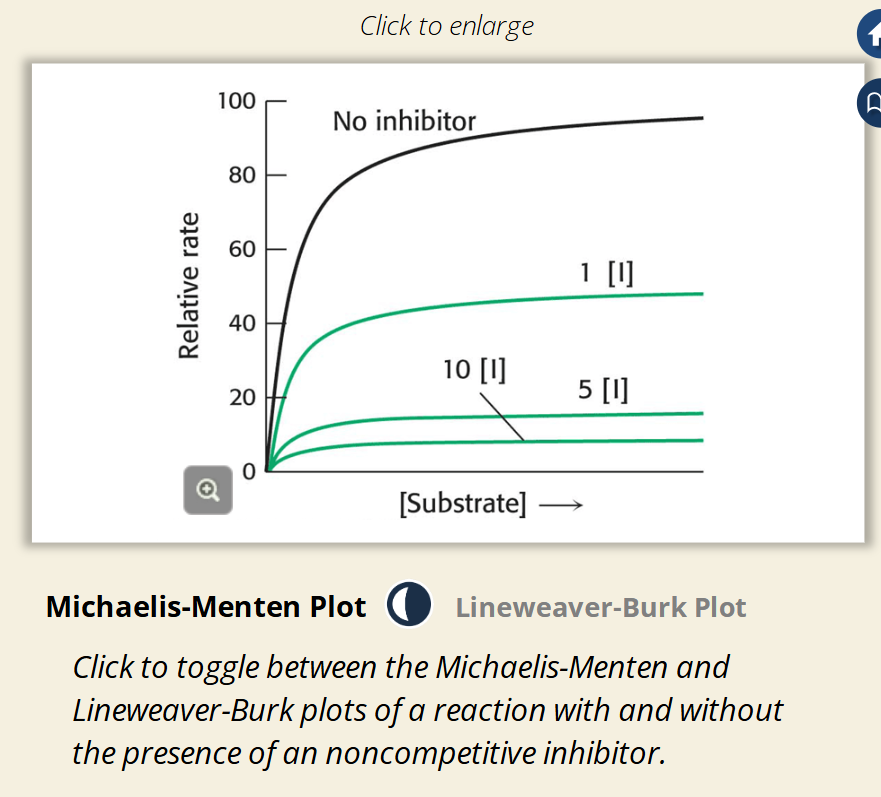
🟢 As inhibitor concentration increases:
• Max rate (Vmax) drops lower
• Curve flattens earlier
📊 All lines still reach the same Km (same x-axis position), but at lower velocities
Flashcard 4: Lineweaver-Burk Plot Explanation (Image 1)
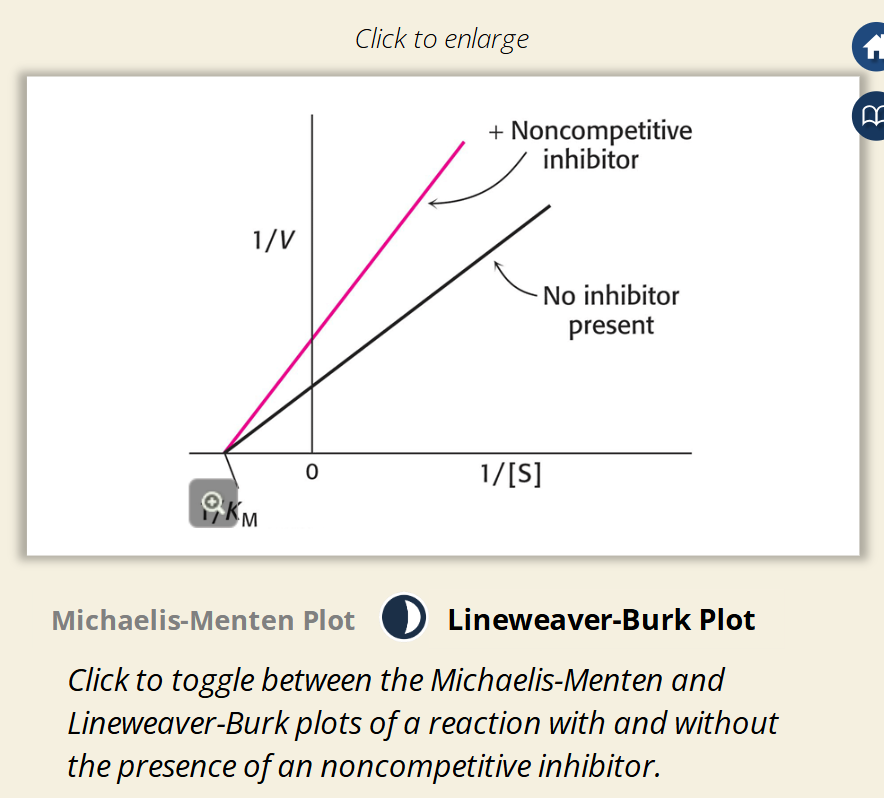
📈 Slope increases (steeper line)
🔹 Y-intercept (1/Vmax) goes up
➖ X-intercept (-1/Km) stays the same
💡 Confirms: Vmax ↓, Km unchanged
Flashcard 5: Key Takeaway
🛑 Noncompetitive inhibitors reduce enzyme efficiency, but don’t interfere with substrate binding
🧪 Used to shut down enzymes without affecting their substrate affinity
Flashcard 1: What is noncompetitive inhibition?
🔒 Inhibitor binds to both:
Free enzyme (E)
Enzyme-substrate complex (ES)
📍 Binding occurs at a separate allosteric site
Flashcard 2: What happens to Km in noncompetitive inhibition?
➖ Km stays the same
📊 Why? Because the inhibitor binds both E and ES equally, keeping substrate binding affinity unchanged
Flashcard 3: What happens to Vmax in noncompetitive inhibition?
📉 Vmax decreases
🧪 Some enzyme is always blocked, so less is available to turn substrate into product
Flashcard 4: Michaelis-Menten equation with noncompetitive inhibitor
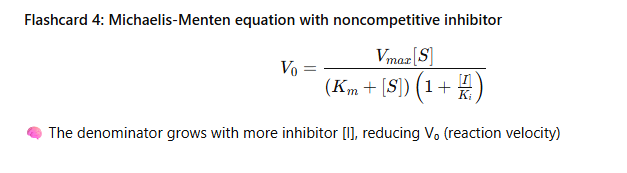
Flashcard 5: Visual interpretation (image)
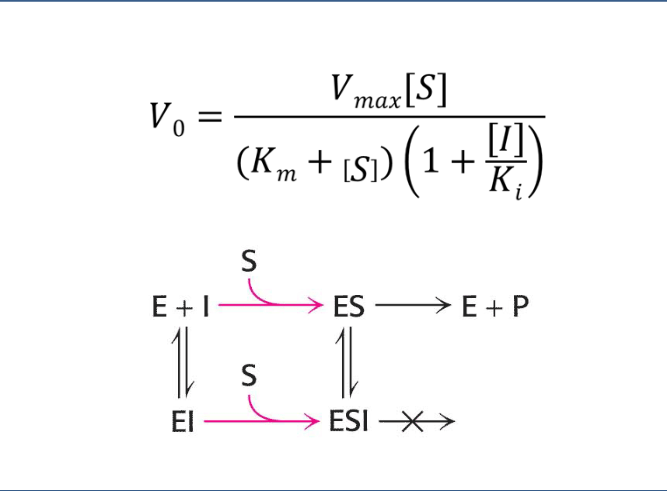
🔁 E + I ↔ EI
🔁 ES + I ↔ ESI (inactive)
🛑 ESI cannot produce product → this reduces the overall reaction rate
Flashcard 1: What are Mixed Inhibitors?
🔀 Bind to both:
Free enzyme (E)
Enzyme-substrate complex (ES)
🧩 Do not follow competitive, uncompetitive, or noncompetitive models exactly
Flashcard 2: Effect on Vmax
📉 Vmax always decreases
🧪 Because some enzyme is always inactivated, regardless of where the inhibitor binds
Flashcard 3: Effect on Km
🔼🔽 Km may increase or decrease
📊 Depends on the specific enzyme-inhibitor interaction
➡ Km behavior is variable in mixed inhibition
Flashcard 4: Summary Rule
Mixed inhibitors:
📉 Decrease Vmax
🔼 or 🔽 Change Km (depends on the system)
Flashcard 1: What do Mixed Inhibitors bind to?
🔗 Mixed inhibitors bind to:
Free enzyme (E)
Enzyme-substrate complex (ES)
Flashcard 2: What effect do Mixed Inhibitors have?
📉 Reduce the enzyme’s ability to:
Bind substrate
Release product
➡ This lowers the number of effective enzymes
Flashcard 3: Can product still be made?
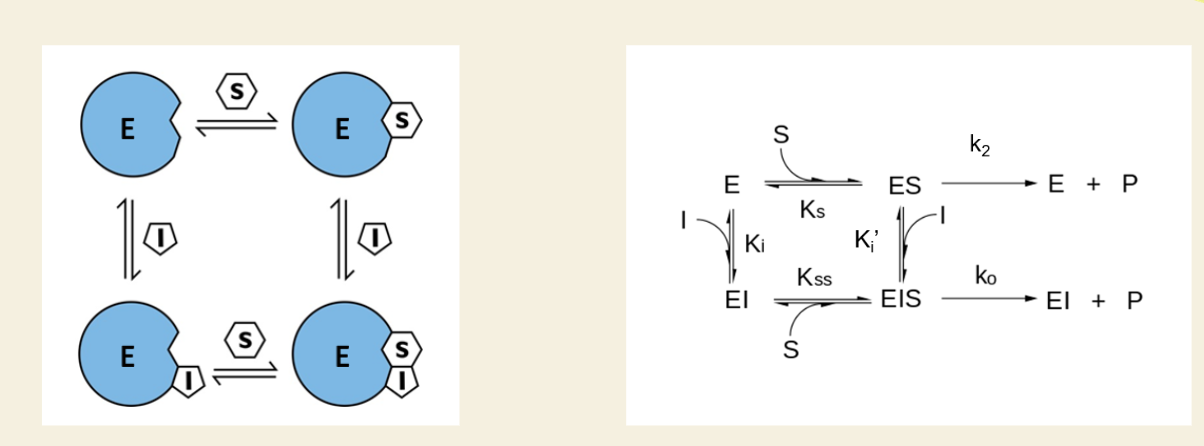
✅ Yes, but at a slower rate
🧪 Product can be made from the EIS complex
📉 This slower rate is represented by k₀
🧠 In the math, this is called β (beta)
Flashcard 4: What’s unique about mixed inhibition vs noncompetitive?
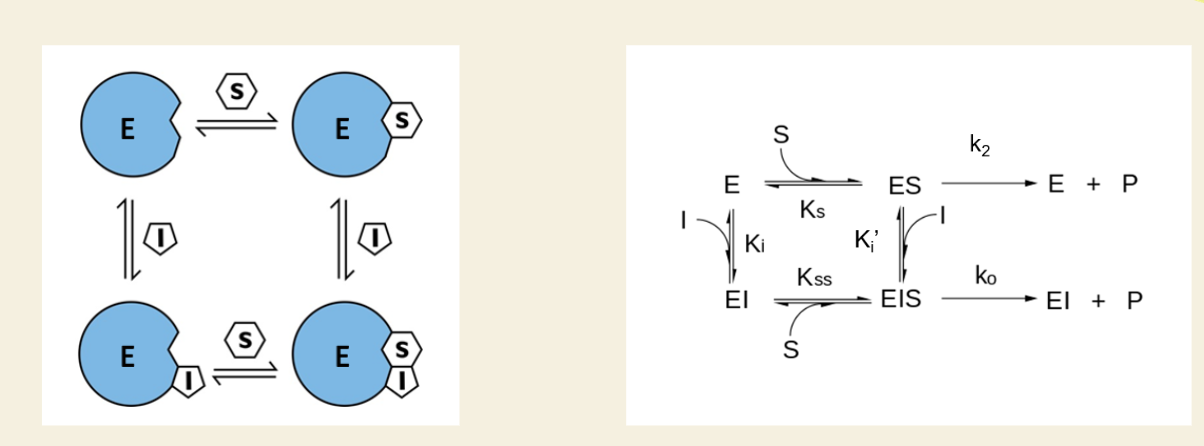
⚖ Noncompetitive = equal affinity for E and ES
🔀 Mixed = different affinity for E and ES
📊 Result: More complex effect on kinetics
Q: What are the effects of mixed inhibitors on enzyme kinetics?
A: Mixed inhibitors always decrease Vmax, and Km may increase or decrease, depending on whether the inhibitor prefers free enzyme or ES complex.
Q: What does it mean when Km is decreased and Vmax is decreased (purple line)?
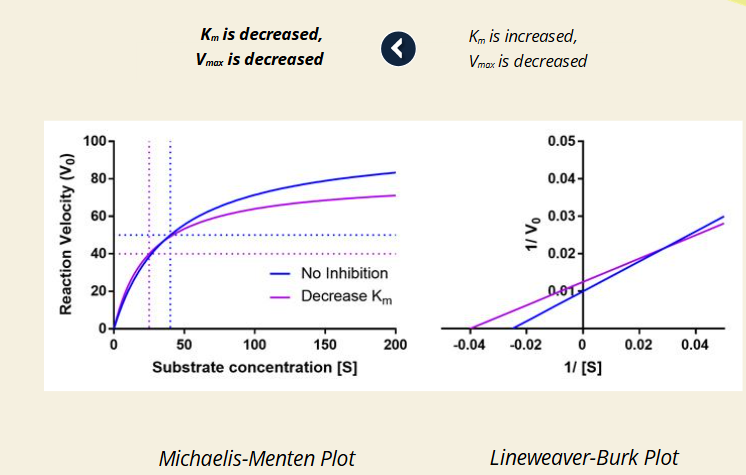
A: The inhibitor prefers the ES complex more.
🔹 This mimics uncompetitive inhibition.
🔹 Line intersects away from axes in Lineweaver-Burk.
Q: What does it mean when Km is increased and Vmax is decreased (red line)?
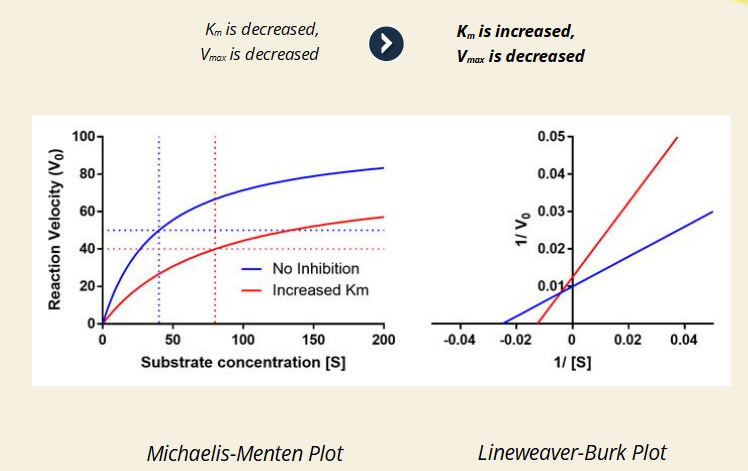
A: The inhibitor prefers the free enzyme more.
🔹 This mimics competitive inhibition.
🔹 Again, the Lineweaver-Burk lines intersect off-axis (not on x or y).
Q: On a Michaelis-Menten plot, how does mixed inhibition appear?
A:
🔸 Vmax shifts lower.
🔸 Depending on Km change, the curve is shifted left (↓Km) or right (↑Km).
Q: On a Lineweaver-Burk plot, how does mixed inhibition appear?
A:
🔸 Lines intersect off-axis (not at the y- or x-intercept).
🔸 Indicates both Km and Vmax are altered.
Q: What is the general equation used when the type of inhibition is unknown?
Q: What does K_i represent in mixed inhibition?
Ki is the affinity of the inhibitor for the free enzyme (E).
Q: What does Ki′ represent in mixed inhibition?
A:
Ki′ is the affinity of the inhibitor for the enzyme-substrate complex (ES).
Q: What does the ratio of Ki′ to Ki tell us?
A:
It tells us how much the inhibitor changes the enzyme’s affinity for the substrate. This ratio helps define the exact inhibition behavior.
Q: Why is the mixed inhibition model useful in experiments?
A:
It provides the best fit when the inhibition mechanism is uncertain or complex, covering multiple types in one formula.
Q: What is another name for irreversible inhibition?
Suicide inhibition — because the enzyme essentially destroys itself by reacting permanently with the inhibitor.
Q: How does irreversible inhibition work?
A:
The inhibitor forms a permanent covalent bond with the enzyme, usually at the active site.
Q: What kind of molecule is an irreversible inhibitor often similar to?
A:
A substrate analogue — it looks like the normal substrate and binds to the active site.
Q: What happens after the enzyme binds the irreversible inhibitor?
The enzyme modifies the inhibitor during the normal catalytic reaction, which activates a reactive group that irreversibly binds to the enzyme.
Q: What is the final result of irreversible inhibition?
A stable enzyme-inhibitor complex is formed, permanently inactivating the enzyme.
Q: What are two examples of drugs that act as irreversible inhibitors?
Aspirin and penicillin.
Q: What does penicillin target in bacteria?
It targets penicillin-binding protein (PBP), which is needed to cross-link peptide chains in the bacterial cell wall.
Q: What is the role of PBP in bacteria?
PBP binds short peptide chains (attached to NAM/NAG sugars) and links them to build a strong cell wall, then dissociates after the job is done.
Q: How does penicillin inhibit PBP?
Penicillin enters the PBP active site and reacts with a critical serine residue needed for enzyme activity.
Q: What happens to penicillin’s β-lactam ring during inhibition?
The β-lactam ring opens irreversibly and forms a covalent bond with PBP, permanently blocking the active site.
Q: Why is penicillin considered an irreversible inhibitor?
Because it forms a permanent covalent bond with the enzyme (PBP), stopping it from ever functioning again.
Q: What kind of drugs are statins and what do they target?
Statins are competitive inhibitors that block HMG-CoA reductase, the enzyme responsible for the rate-limiting step in cholesterol synthesis.