CHEM 1150: Final Exam Review
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
108 Terms
diatomic elements
H2 , O2, N2 , F2 , Cl2 , Br2 , I2
how to find the # of empirical formulas
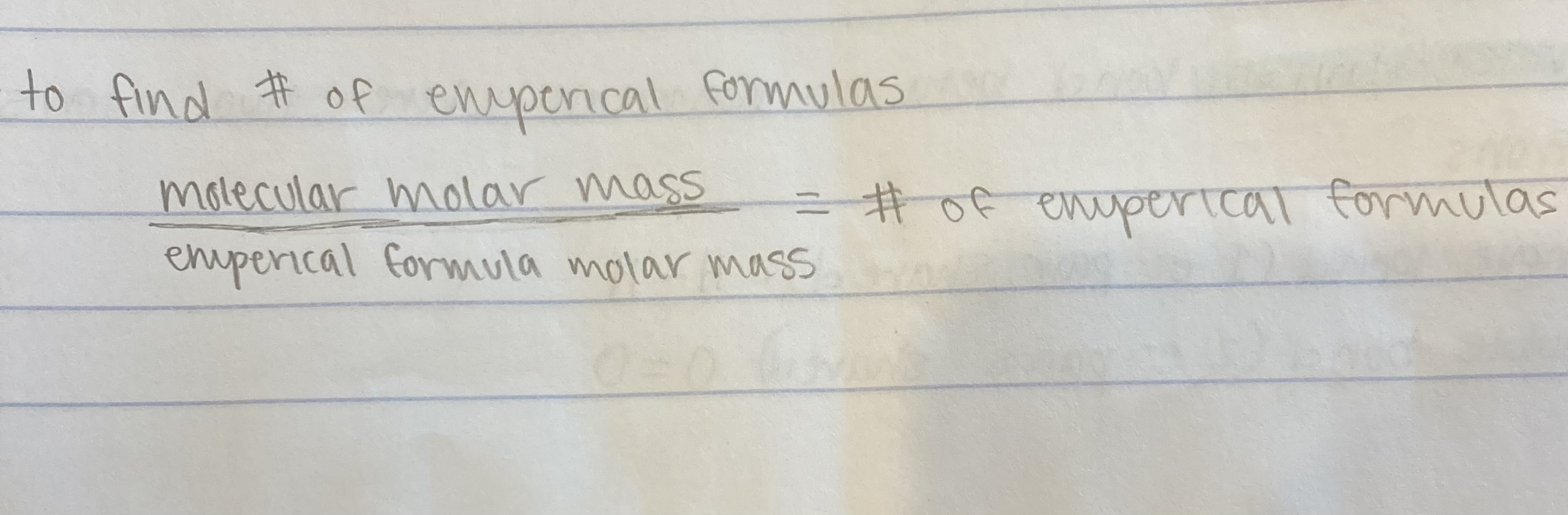
alpha (α) particle
4 2He2+ is emitted from the nucleus
A= decreases by 4, Z=decreases by 2
beta (β⁻) particle
0-1e an electron emitted from a nucleus when a neutron converts into a proton and a positron
A = unchanged, Z = increase by 1
positron (β⁺)
positively charged “electron” emitted from a nucleus when a proton → neutron & β⁺
A = unchanged, Z = decrease by 1
electron capture
0-1e one of the isotope’s own electrons crashes into the nucleus, converting a proton to a neutron
A = unchanged, Z= decrease by 1
critical mass ( of fissionable material)
the amount of the isotope needed to self sustain a chain reaction once it’s initiated
if there isn’t enough material, you have a subcritical mass
radioactive tracers
compounds that have a stable element in a molecule with its radioactive version
movement is tracked by the radiation emitted
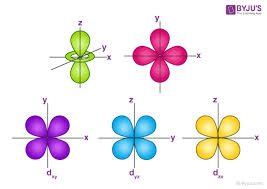
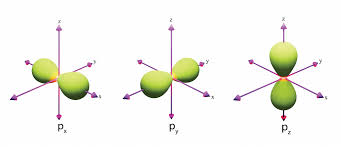
𝑙 = angular momentum quantum #
characterizes the shape and type of orbital
𝑙 = 0 → s
𝑙 = 1 → p
𝑙 = 2 → d
𝑙 = 3 → f
𝑚𝑙 = magnetic quantum #
characterizes the orientation of the orbital (and how many of each orbital type exist)
𝑚𝑙 = - 𝑙, -𝑙 +1 … -1, 0, 1. . . 𝑙 - 1, 𝑙
Helium [He] Nobel Gas Configuration
1s2 (2 electrons)
Neon ([Ne] Noble Gas Configuration
1s22s22p6 (10 electrons)
Argon [Ar] Noble Gas Configuration
1s22s22p6 3s23p6 (18 electrons)
Krypton [Kr] Noble Gas Configuration
1s22s22p6 3s23p6 4s2 d104p6 (36 electrons)
Xenon [Xe] Noble Gas Configuration
1s22s22p63s23p6 4s2 3d104p6 5s2 4d10 5p6 (54 electrons)
Radon [Rn] Noble Gas Configuration
1s2 2s2 2p6 3s23p6 4s2 3d104p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6 (86 electrons)
ionization energy
energy required to remove an electron from an atom
electron affinity
energy change when an electron is added to an atom
Lewis Structure Rules
H is always on the outside of a molecule (can only form one bond)
C is always the central atom
Otherwise, the central atom will have the lowest electronegativity

LEONA
loses electrons oxidation negative anode
GERPC
gains electrons reduction postive cathode
how to determine the anode
the metal higher in the EMF series, which is donating electrons
how to determine the cathode
the metal lower in the EMF series, which is accepting electrons
percent yield
actual amount of product/theoretical yield of product x 100%
enthalpy
a value (H) which gives a measure of energies related to molecules (such as heat energy “stored” in a molecule in bonds) and processes (such as chemical reactions)
ΔHrxn = H of products - H of reactants
if ΔHrxn < 0, reactants had a higher total enthalpy than products
if ΔHrxn > 0, products had a higher total enthalpy than reactants
reaction mechanism
the sequence of steps molecules go through as they go from reactants → products
the slowest rate-limiting step will control the rate of the overall reaction
collision theory
two molecules must collide with an energy greater than/equal to the activation energy
molecules must also collide in the proper spatial orientation so that right bonds can break/form between the right atoms
reaction rate
can be thought of as a function of molecular energy (of collision) & molecular orientation
reaction rate = (# of molecular collisions per unit time/energy ≥ Ea) x (proportion of collisions in which the colliding molecules are in the correct orientation)
concentration and reaction rates
the higher the # of molecules of the reactants, the more total collisions would occur, making the reaction faster
temperature and reaction rates
the higher the temperature, the faster molecules move
the average speed of a molecule depends on the temperature the molecule is at & the molecular weight
rate constant
rate = (k) x (total # of collisions per unit time)
is temperature dependent
is used to quantify all things in a reaction that we can’t control
plausibility of mechanisms
for a proposed mechanism to be plausible, the experimentally measured rate law for the overall reaction must match the rate law of the proposed rate limiting step
catalyst
a substance that participates in a reaction, but isn’t permanently changed by the reaction
it is a reactant, but gets returned as a product in a later step
types of catalysts
homogenous: a substance mixed in with the reactants
heterogenous: a surface upon which reactants absorb, react, and release from as a new species
enzymes: biological catalysts
enzymes
large biomolecules w/ specific active sites that allow an enzyme to selectively interact w/ a specific substrate molecule
chemical equilibrium
equilibrium is established when the rate of the forward reaction = the rate of the reverse reaction
at equilibrium, reactants are forming products while products are forming reactants, and the amounts don’t change
rate = kforward [A]eq [B]eq = kreverse [W]eq [X]eq
keq depends on the equilibrium concentrations of all species and their stoichiometric coefficients in the overall reaction
equilibrium constant
for a general equilibrium reaction (at a given T)
a A + b B ⇌ c C + d D
Keq = [C]eq c x [D]eq d / [A]eq a x [B]eq b
-if Keq is large ( > 103), [products] > [reactants]
-if Keq is small ( < 10-3), [products] < [reactants]
- if Keq is moderate (10-3 < Keq < 103 )
reaction quotient
if we mix concentrations of substances, we can know which way the reaction will go to reach equilibrium
Q = [C]actual c x [D]actual d / [A]actual a x [B]actual
- if Q < Keq, the reaction will make more product
- if Q > Keq, the reaction will make more reactant
- if Q = Keq, the reaction is already at equilibrium
le chatelier’s principle
if a reaction at equilibrium is disturbed, the reaction will shift direction as necessary to counteract the disturbance
a A + b B ⇌ c C + d D
- add A or B or remove C and D = reaction will shift right toward products
- remove A or B or add C and D = reaction will shift left toward reactants
temperature and equilibrium
for an exothermic (ΔHrxn < 0) reaction
reactants ⇌ products + heat
- raising the temp will shift the reaction to the left to “consume” some of the added heat
- lowering the temp removes heat, reaction will shift to the left to produce more
for an endothermic (ΔHrxn > 0) reaction
reactants + heat ⇌ products
- raising the temp will shift the reaction to the right to “consume” some of the added heat
- lowering the temp removes heat, reaction will shift to the left to produce more
arrhenius acids and bases
acids: dissociate in water to form H+
HA (aq) → H+ (aq) + A- (aq)
bases dissociate in water to form OH-
MOH (aq) → M+ (aq) + OH- (aq)
weak acid equilibrium
Ka = [H3O+]eq x [A-]eq / [HA]eq
Bronsted-Lowry definition of an acid
a substance that donates a proton
Bronsted-Lowry definition of an base
substance that accepts a proton
water auto-ionization constant
Kw = 1 × 10-14
when is a solution acidic?
[H3O+] > 1 × 10-7 moles/L, [OH-] < 1× 10-7 mol/L