Biology- Unit 2A: Chemistry of Life (Atomic Structure and Bonds, Properties of Water, and pH and Buffers)
1/41
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Atom
The smallest unit of matter.
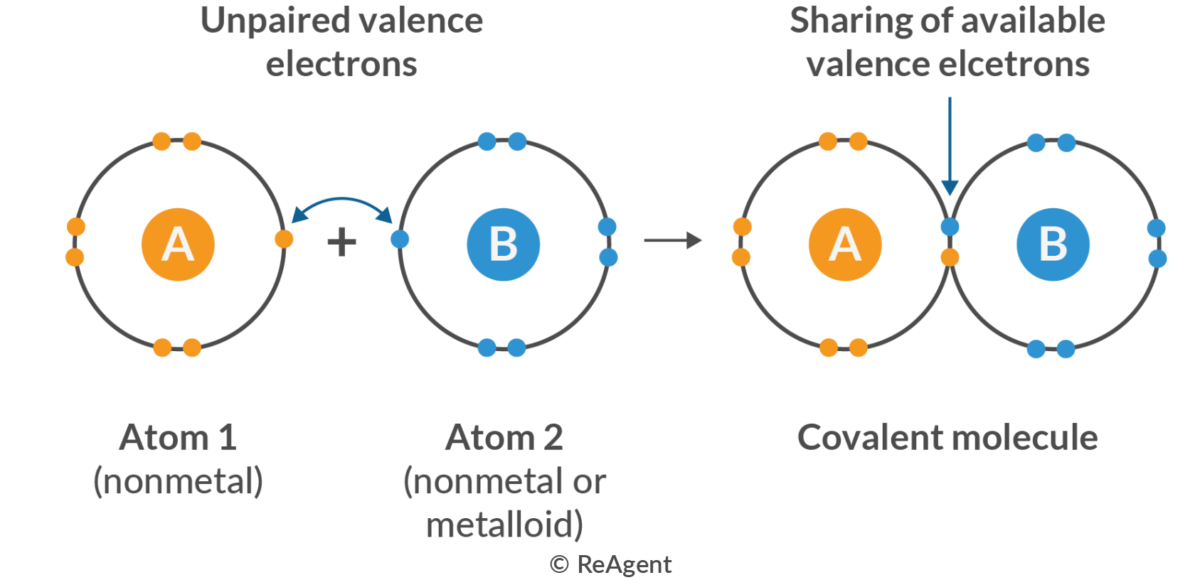
Covalent Bond
The type of bond formed when two atoms share a pair of electrons.
Proton
The particle in the nucleus of an atom that carries a positive charge.
Neutron
The particle in the nucleus of an atom that carries no charge.
Atomic Mass
Indicates the number of protons plus the number of neutrons in an atom.
Electron
The particle that moves around the nucleus of an atom and carries a negative charge.
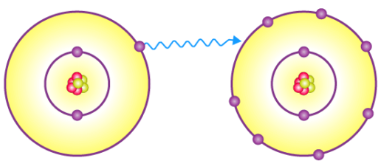
Ionic Bond
The type of bond formed through electrical attraction between oppositely charged ions.
Ion
An atom that has gained or lost one or more electrons.
Atomic Number
This indicates the number of protons in an atom.
Valence Electrons
The electrons in the outermost energy level of an atom.
CaCl2 is a __ bond.
Ionic - metal + nonmetal
K2SO4 is a __ bond.
Ionic - metal + nonmetal
ZnO is a __ bond.
Ionic - metal + nonmetal
C6 H14 is a __ bond.
Covalent - nonmetal + nonmetal
CH4 is a __ bond.
Covalent - nonmetal + nonmetal
CH3COCH3 is a __ bond.
Covalent - nonmetal + nonmetal

Imbibition
Allows seeds to grow in order for plants to grow and develop.

Cohesion
Allows water to join with other water molecules.

Adhesion
Allows water to join with other substances.
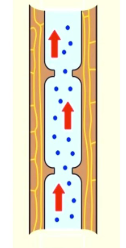
Capillary Action
Allows plants to obtain the necessary water for photosynthesis from its roots to its leaves.

Surface Tension
Allows organisms, like water striders (insects) to walk on water.

Universal Solvent
Allows nutrients to dissolve in water so organisms can obtain them.

High Heat Capacity
Allows organisms that live in the water a constant living environment.
Acid
An __ is a compound that contains more H+ ions in a solution.
Buffer
A __ is a substance that resists changes in pH.
Base
A __ is a compound that contains more OH- ions in a solution.
Neutral
A __ solution contains equal concentrations of H+ and OH- ions.
A solution with a pH of __ would be considered neutral (0-6.99; 7; or 7.01-14)
7
A solution with a pH of __ would be considered an acid (0-6.99; 7; or 7.01-14)
0-6.99
A solution with a pH of __ would be considered a base (0-6.99; 7; or 7.01-14)
7.01-14
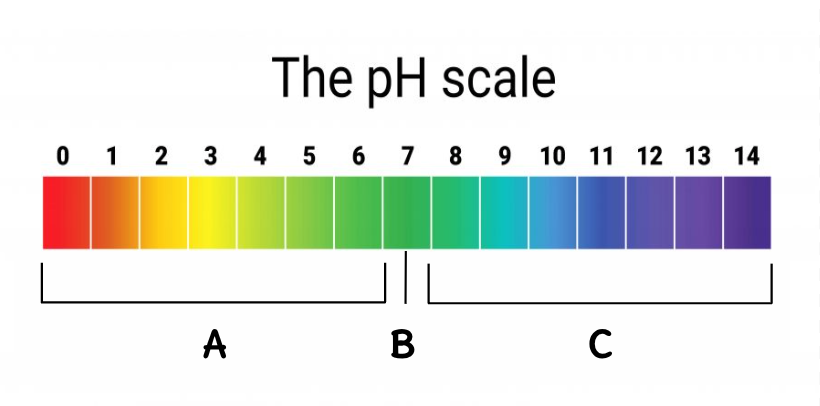
Where are the acids located on this scale?
A
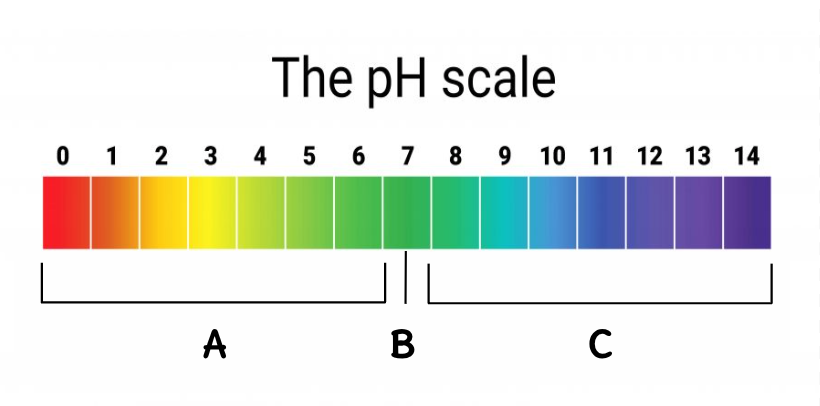
Where is the neutral located on this scale?
B
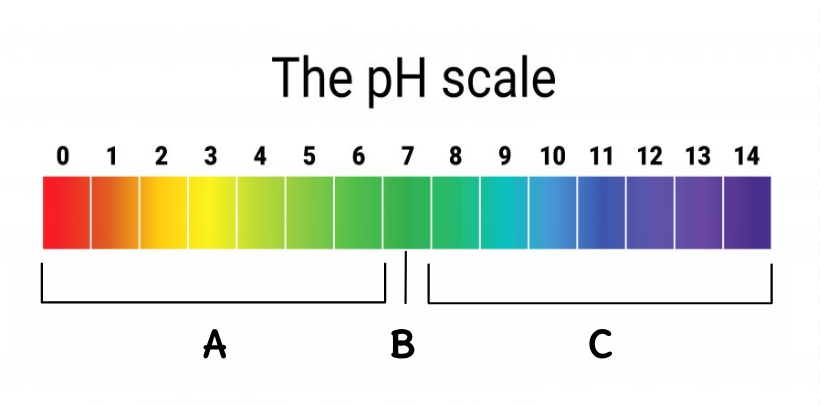
Where are the bases located on this scale?
C
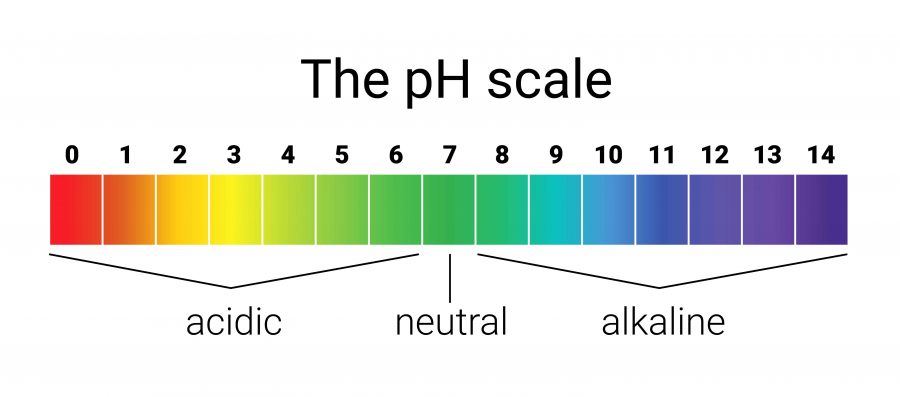
Which side has the MOST H+ ions and the LEAST OH- ions?
Acidic Side
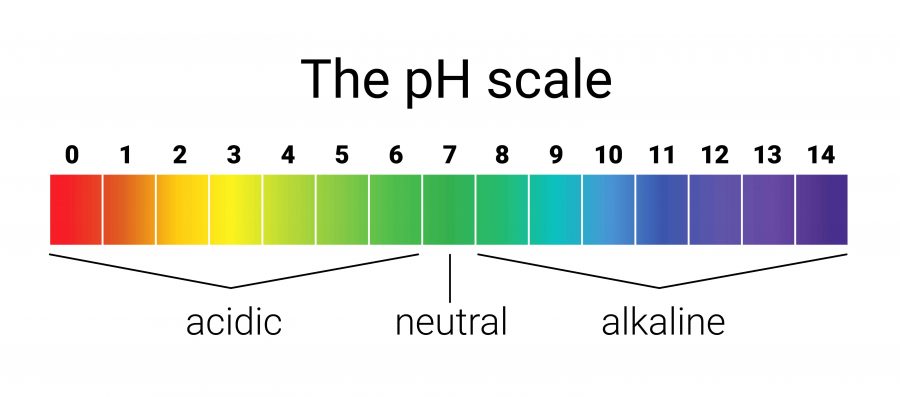
Which side has the LEAST H+ ions and the MOST OH- ions?
Basic Side
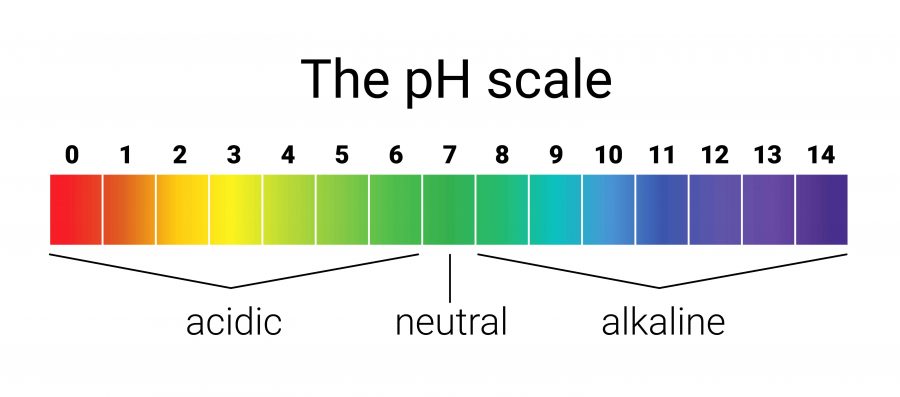
Which side has EQUAL amounts of H+ ions and OH- ions?
Neutral Middle
The strongest acid is the substance w/ a pH closest to __.
0
The strongest base is the substance w/ a pH closest to __.
14
The weakest acid is the substance w/ a pH closest to __.
6
The weakest base is the substance w/ a pH closest to __.
8
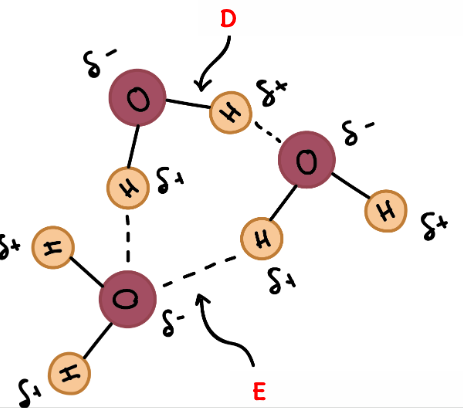
What is D an example of?
A Polar Covalent Bond
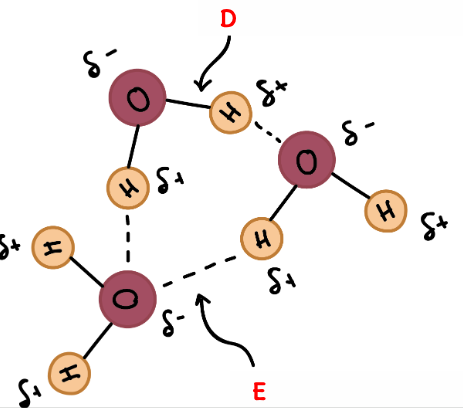
What is E an example of?
A Hydrogen Bond