AP Biology - Functional Groups(and 6 other thingies)
0.0(0)
0.0(0)
New
Card Sorting
1/13
Earn XP
Description and Tags
Honestly just stuff I got from an Edpuzzle. Still, should be some help to ya.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
1
New cards
Organic Chemistry
The study of carbon-containing compounds.
2
New cards
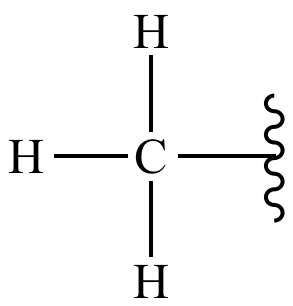
Four valence electrons
Carbon has ____________, making it able to have four covalent bonds at the same time.
3
New cards
Isomers
Molecules with the same formula but different structures are called ______, as they can completely change the function of a molecule.
4
New cards
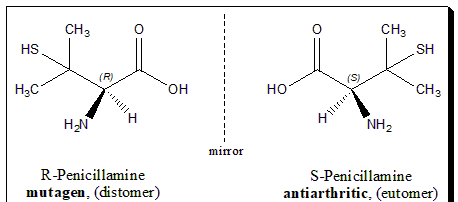
Enantiomers
A sub-category of isomers; essentially mirror-images of the original molecule.
5
New cards
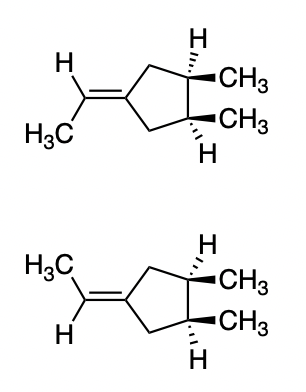
Geometrical Isomers
A sub-category of isomers; where the atoms are arranged differently, but with the same bonds as the original.
6
New cards
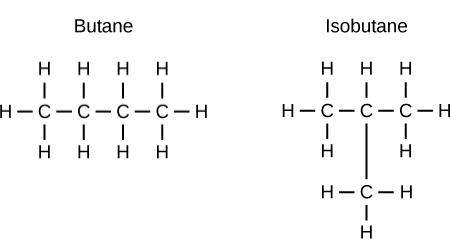
Structural Isomers
A sub-category of isomers; when atoms are bonded to different atoms in comparison to ther original.
7
New cards
Functional Groups
These different GROUPS of different atoms/molecules give compounds and macromolecules their different properties, or FUNCTIONS.
8
New cards
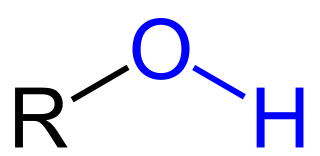
Hydroxyl
\-Polar, Hydrophilic
\-good for reactions
\-Becomes an alcohol when R is a alkyl group
\-good for reactions
\-Becomes an alcohol when R is a alkyl group
9
New cards
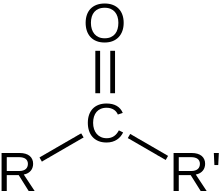
Carbonyl
\-Common in carbohydrates
\-Not to be confused with group of similar name
\-Not to be confused with group of similar name
10
New cards
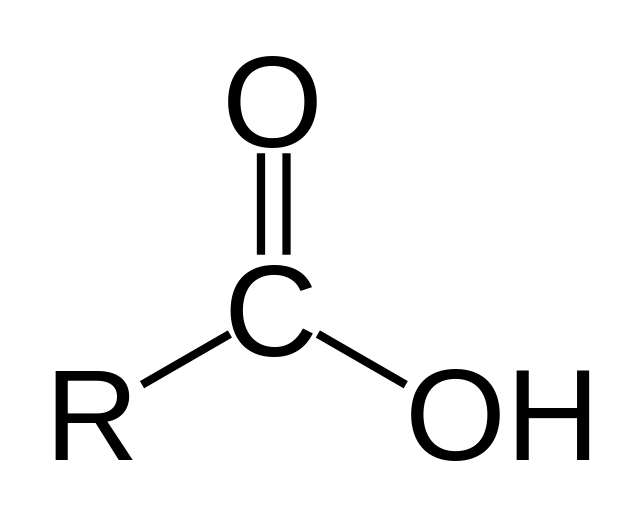
Carboxyl
\-Acidic, attached to amino ACIDS
\-Not to be confused with group of similar name
\-Not to be confused with group of similar name
11
New cards
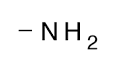
Amino
\-Basic
\-Compounds are amines
\-Attached to AMINO acids
\-Compounds are amines
\-Attached to AMINO acids
12
New cards
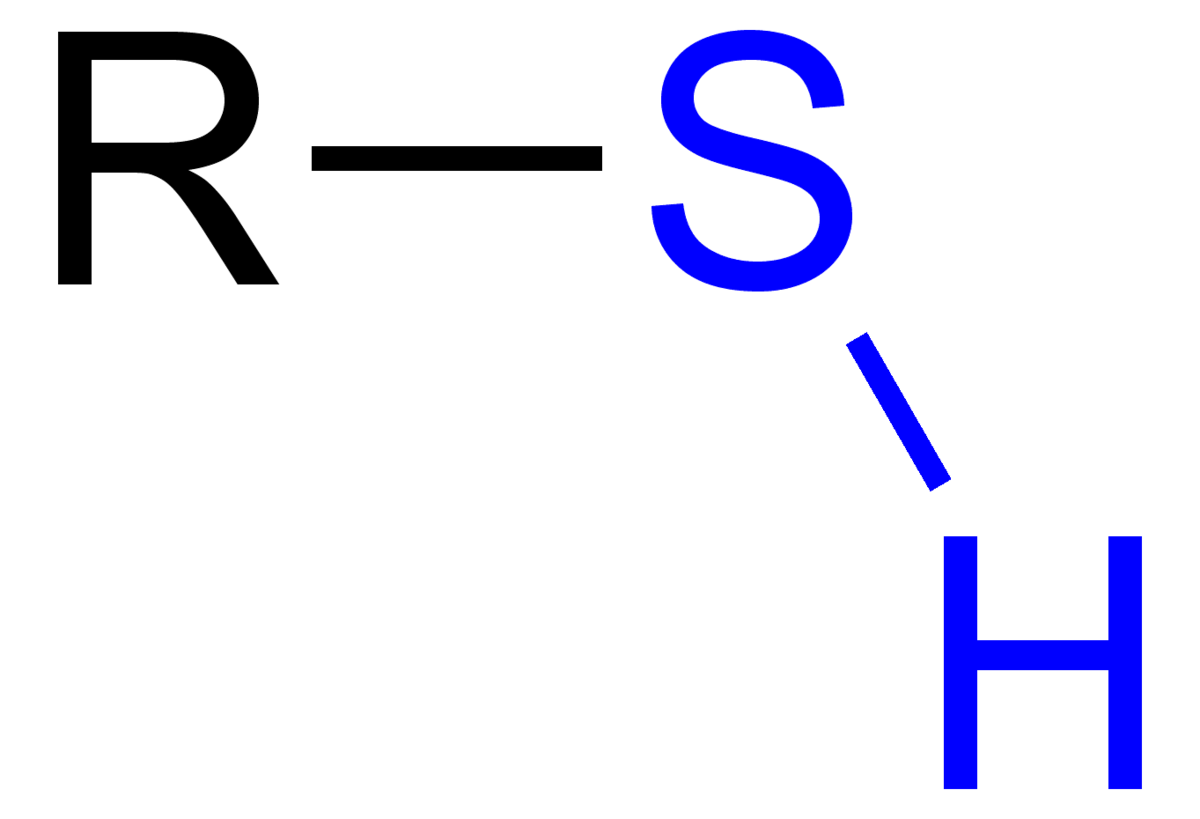
Sulfhydryl
\-Also referred to as a thiol group
\-Compounds are thiols
\-stabilizes some proteins, so sometimes found in amino acids
\-smells really bad
\-Compounds are thiols
\-stabilizes some proteins, so sometimes found in amino acids
\-smells really bad
13
New cards
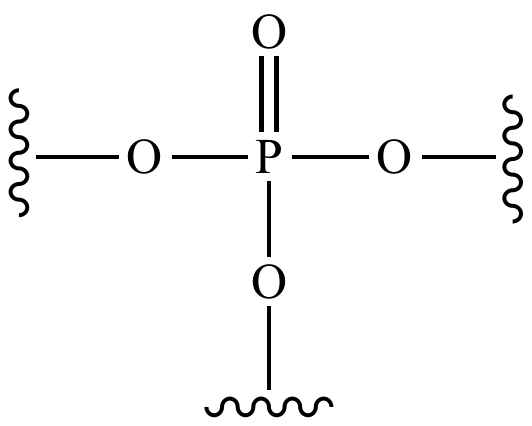
Phosphate
\-good for energy transfer
\-found in ATP(it’s the P, by the way) and special lipids for the cell membrane
\-found in ATP(it’s the P, by the way) and special lipids for the cell membrane
14
New cards
Methyl
\-Super important for gene expression, found in nucleic acids
\-hydrophobic, so found in lipids, too
\-hydrophobic, so found in lipids, too