Carboxylic Acids and Esters: Key Concepts and Reactions
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Carboxylic Acid
Organic compound containing a carboxyl group (-COOH).
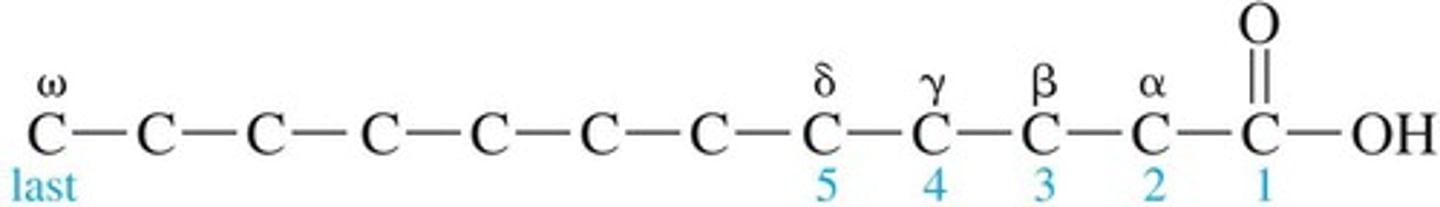
Carboxyl Group
Functional group characteristic of carboxylic acids.
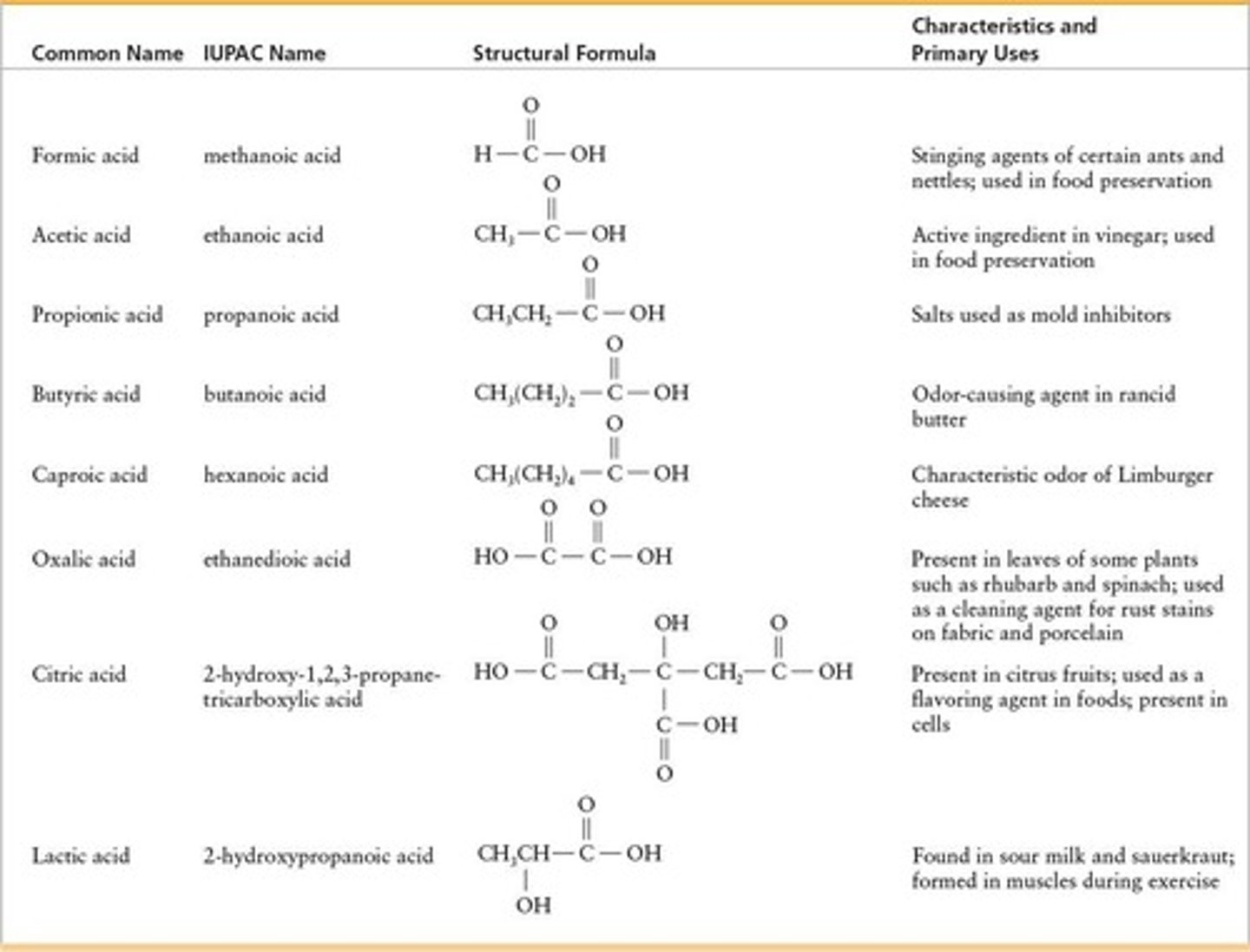
IUPAC Nomenclature
Systematic naming of chemical compounds using specific rules.
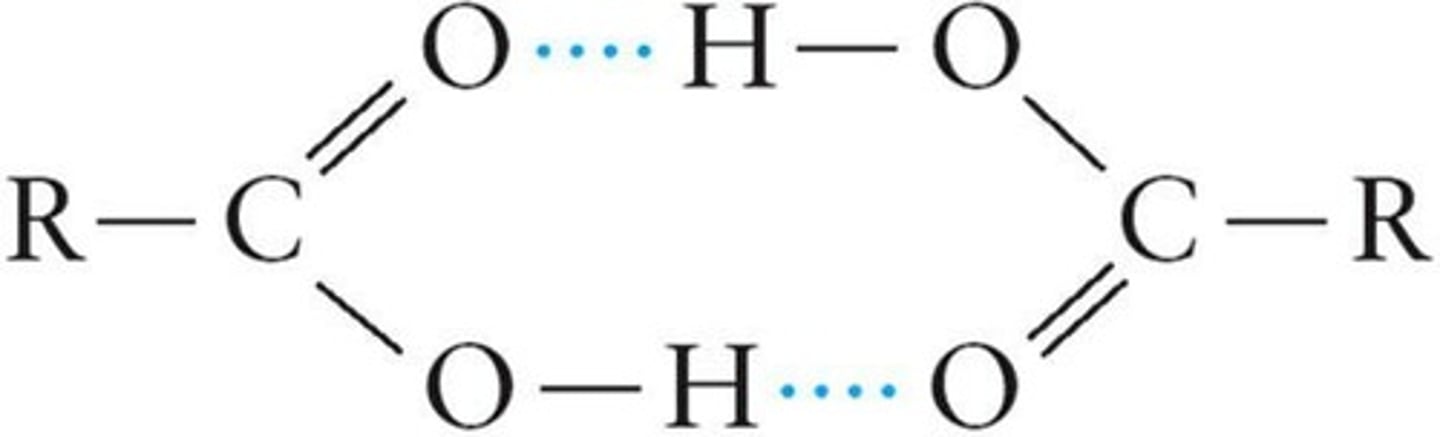
Dimers
Two identical molecules bonded together.
Hydrogen Bonding
Attraction between hydrogen and electronegative atoms.
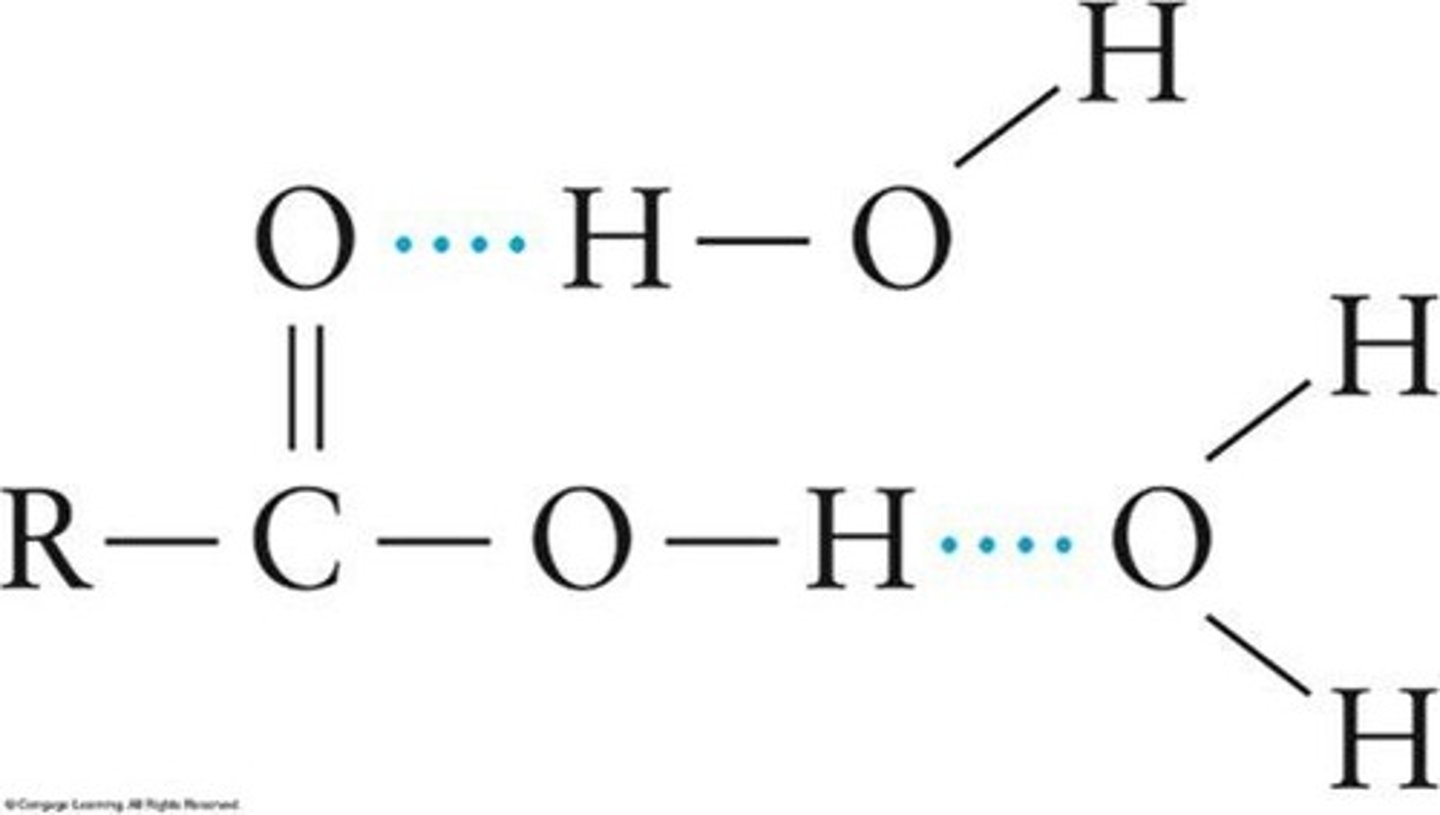
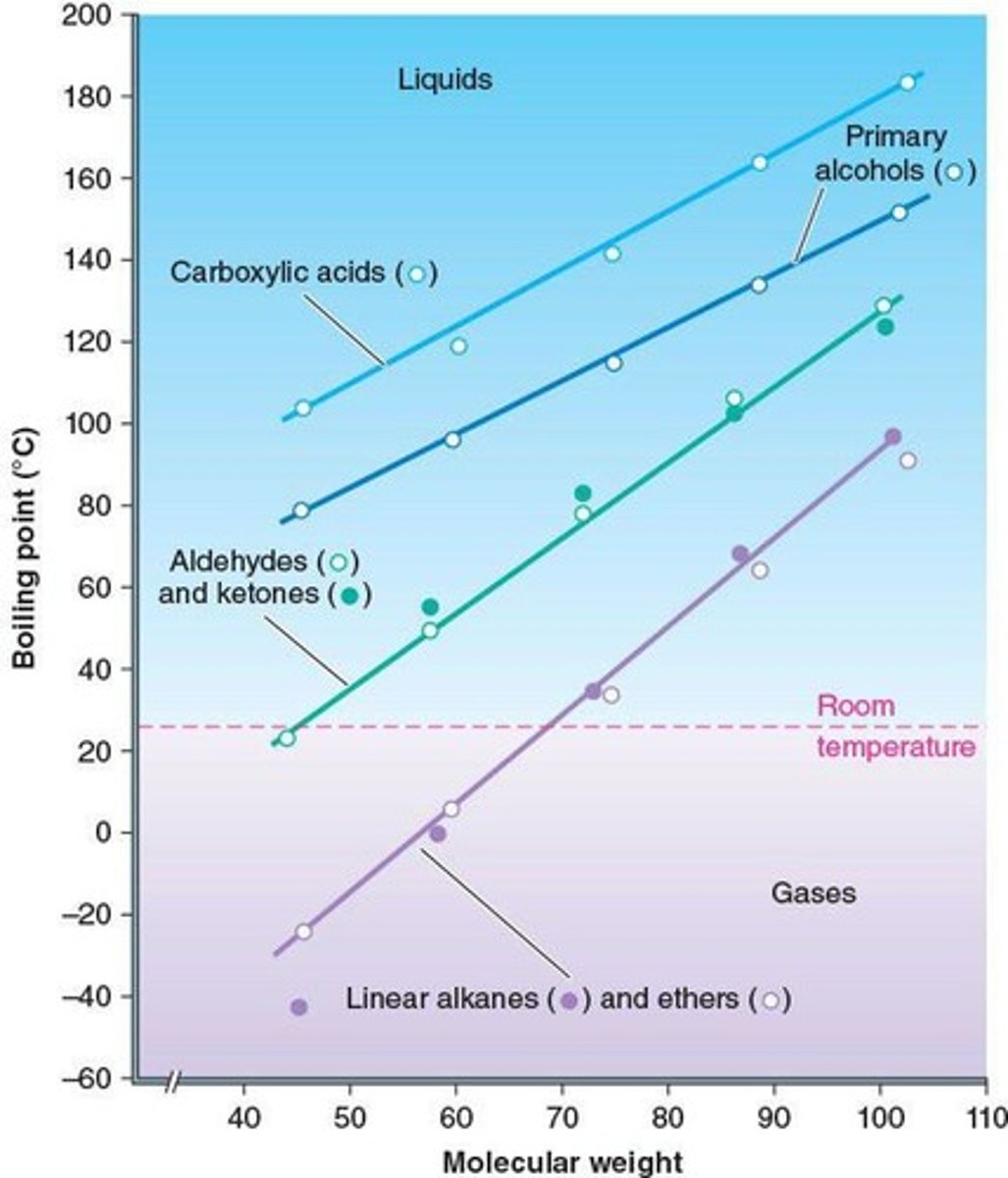
Boiling Point
Temperature at which a liquid turns to vapor.
Solubility
Ability of a substance to dissolve in water.
Fatty Acids
Carboxylic acids with long hydrocarbon chains.
Esterification
Process of forming an ester from acid and alcohol.
Ester Linkage
Bond between carbonyl carbon and oxygen in esters.
Polyester
Polymer formed through esterification process.
Saponification
Alkaline hydrolysis of esters producing soap.
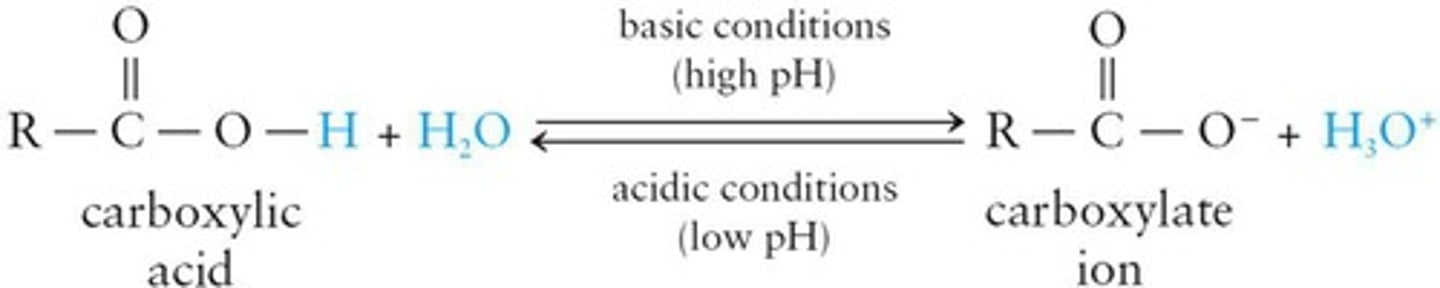
Carboxylate Ion
Anion formed from carboxylic acid by deprotonation.
Trans Fats
Unsaturated fats with trans double bonds, unhealthy.
Hydrophobic R Group
Non-polar hydrocarbon chain affecting solubility.
Aliphatic Carboxylic Acids
Carboxylic acids with straight or branched chains.
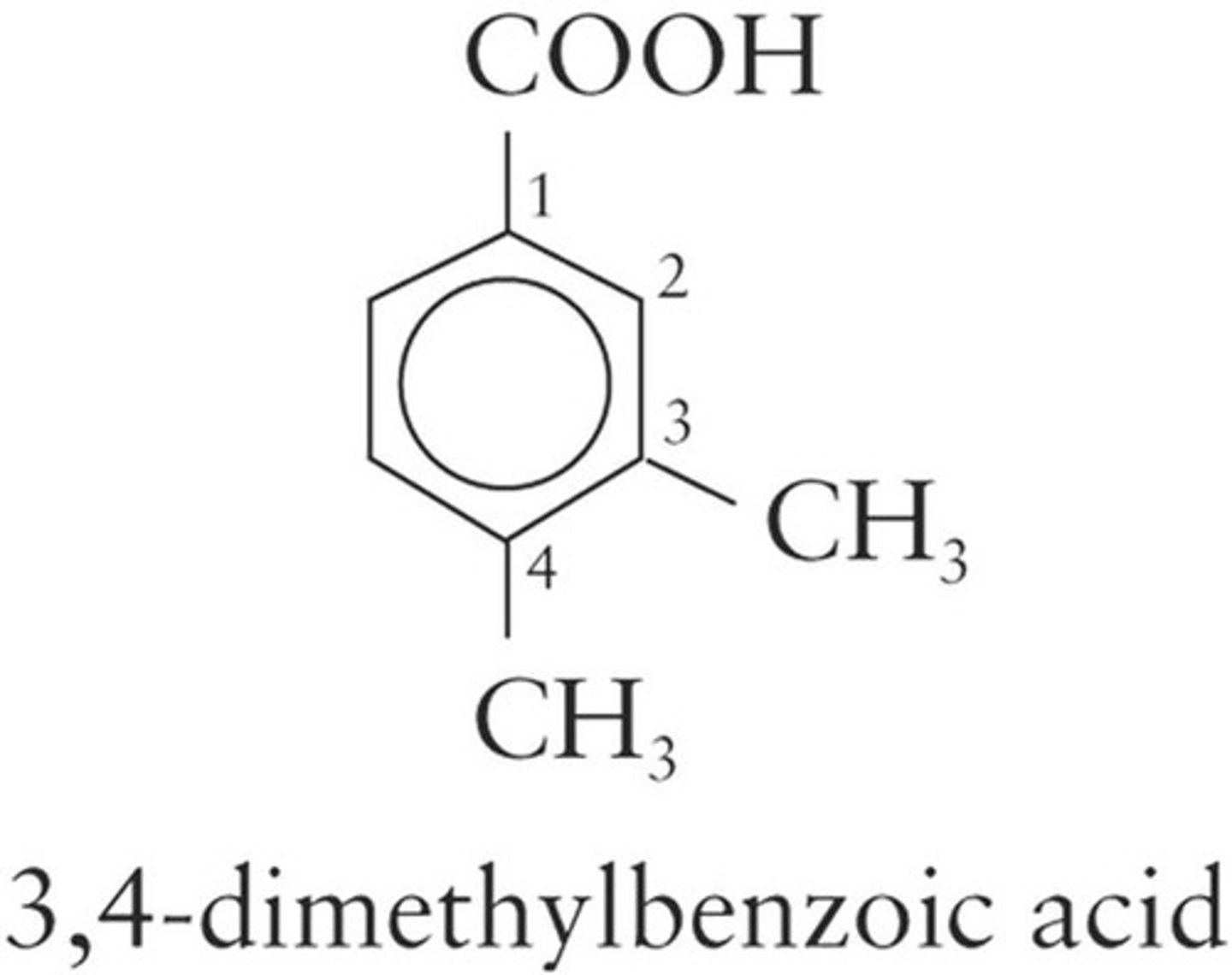
Aromatic Acids
Carboxylic acids derived from aromatic compounds.
Oxidation
Chemical reaction involving loss of electrons.
Primary Alcohols
Alcohols with the hydroxyl group on a terminal carbon.
Condensation Polymerization
Monomers combine with elimination of small molecules.
Ester Hydrolysis
Reaction of ester with water to produce acid and alcohol.
Phosphate Esters
Esters formed from phosphoric acid and alcohols.
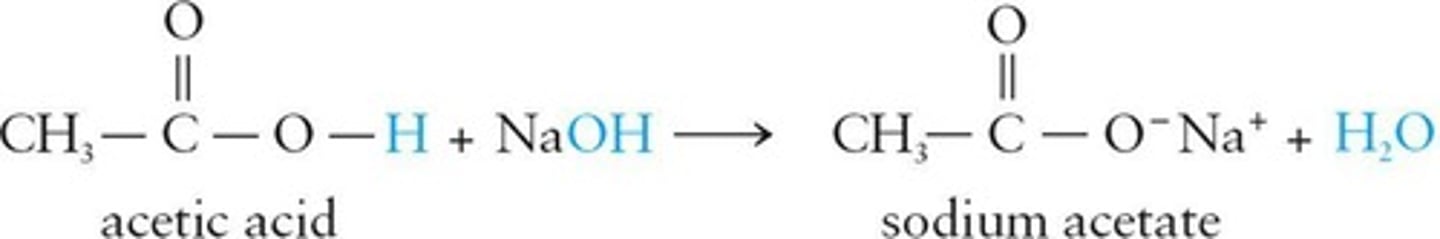
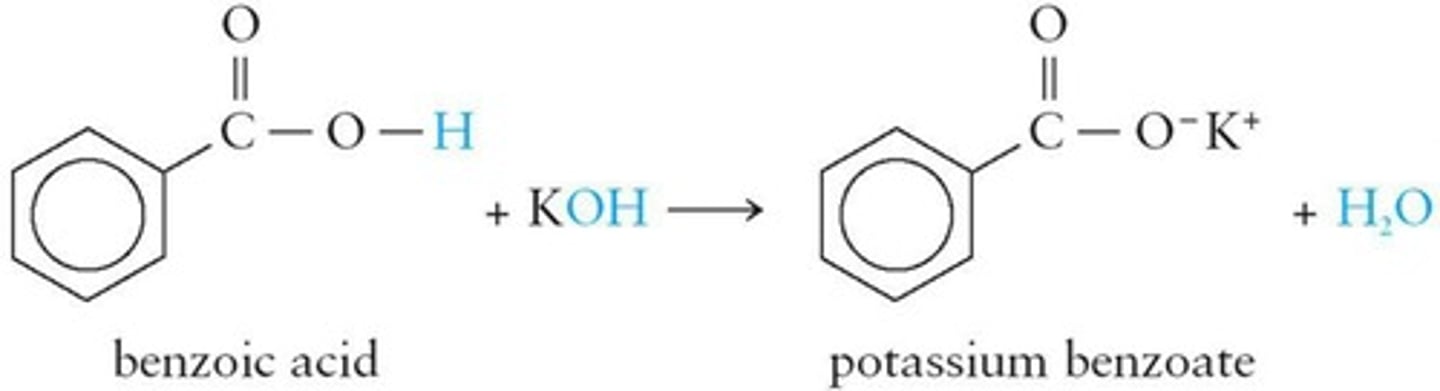
Carboxylic Acid Salts
Salts formed from carboxylic acids and bases.
Common Names
Traditional names used for carboxylic acids.
Hydrogenation
Addition of hydrogen to unsaturated fats.
Biological Importance
Significance of compounds in biological processes.
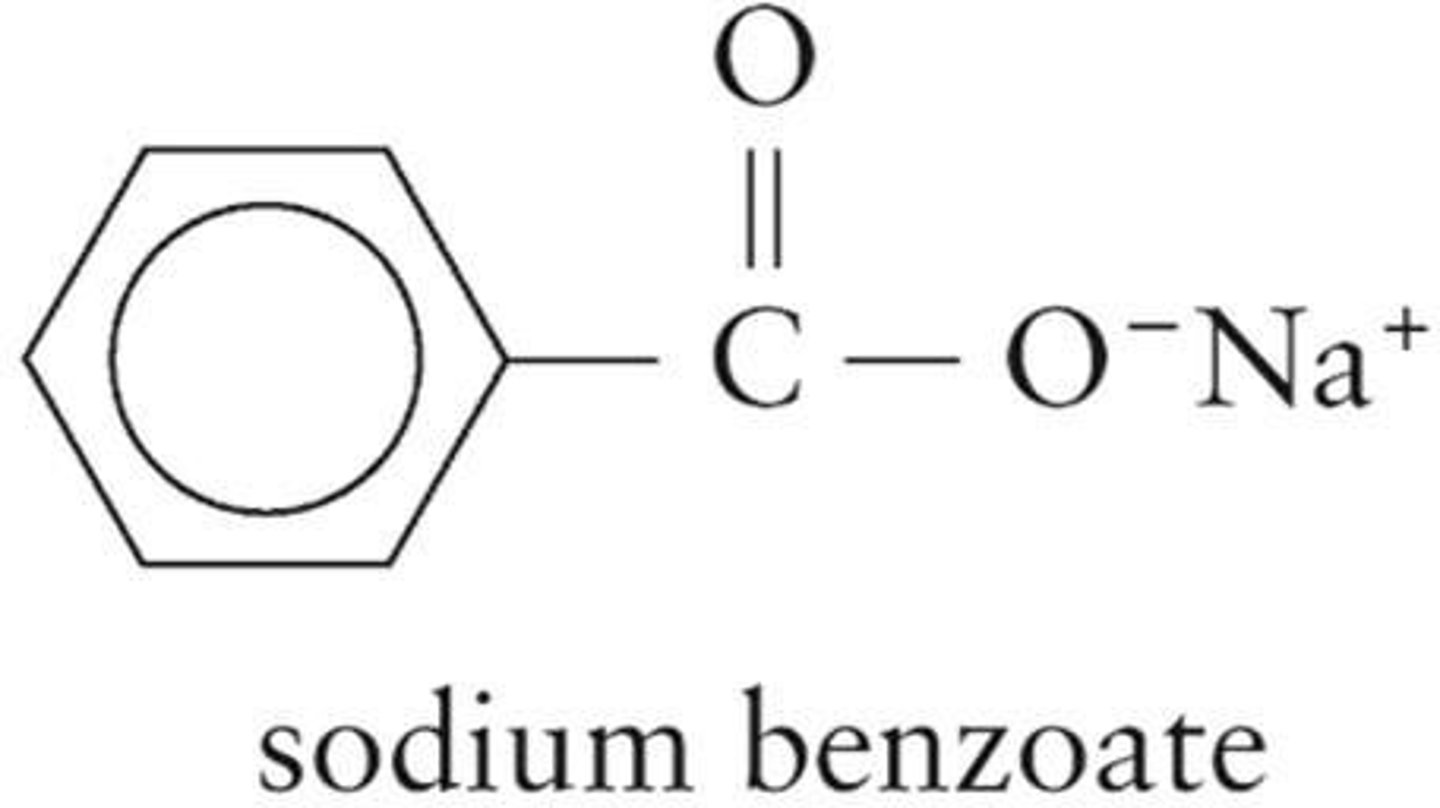
Sodium Benzoate
Food preservative derived from benzoic acid.
Calcium Propanoate
Preservative used in bakery products.
Methyl Acetate
Common ester used as a solvent.