HN220 Unit 1
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
Homeostasis
The central organizing principle of physiology (ie. Walter Cannon)
- does not imply that something is immobile, but as a condition that may fluctuate/vary, but remain relatively constant
What are the Components of Homeostatic System?
1. Regulated variable
2. Set point
3. Sensory
4. Integrating centre
5. Effectors
**negative feedback loop from effectors
Regulated variable
ie. blood pressure, temperature, insulin levels, pH, heart rate, etc.
Set Point
Every regulated variable has this
(ie. blood temp is 37.5)
Any difference between the actual value and the set point constitutes an error signal
Sensor
Cells (neurons) that are sensitive to the variable in question; relay signals to integrating centre
ie. thermoreceptor (skin, brain, central, peripheral)
Integrating centre
Receive sigmals (input) from sensory, which differs from the set point and ilicits an appropriate signals (output) to cells, tissues, or organs
ie. in this example is the hypothalamus (temperature from the skin, central signal receptors)
Effectors
Organs that can exert a change (received from an integrating centre)
ie. Arterioles - level of vascular at which you can regulate and control flow, vasodilate, increase covective heat loss, or Glands - increase sweat, increase evaporative heat loss
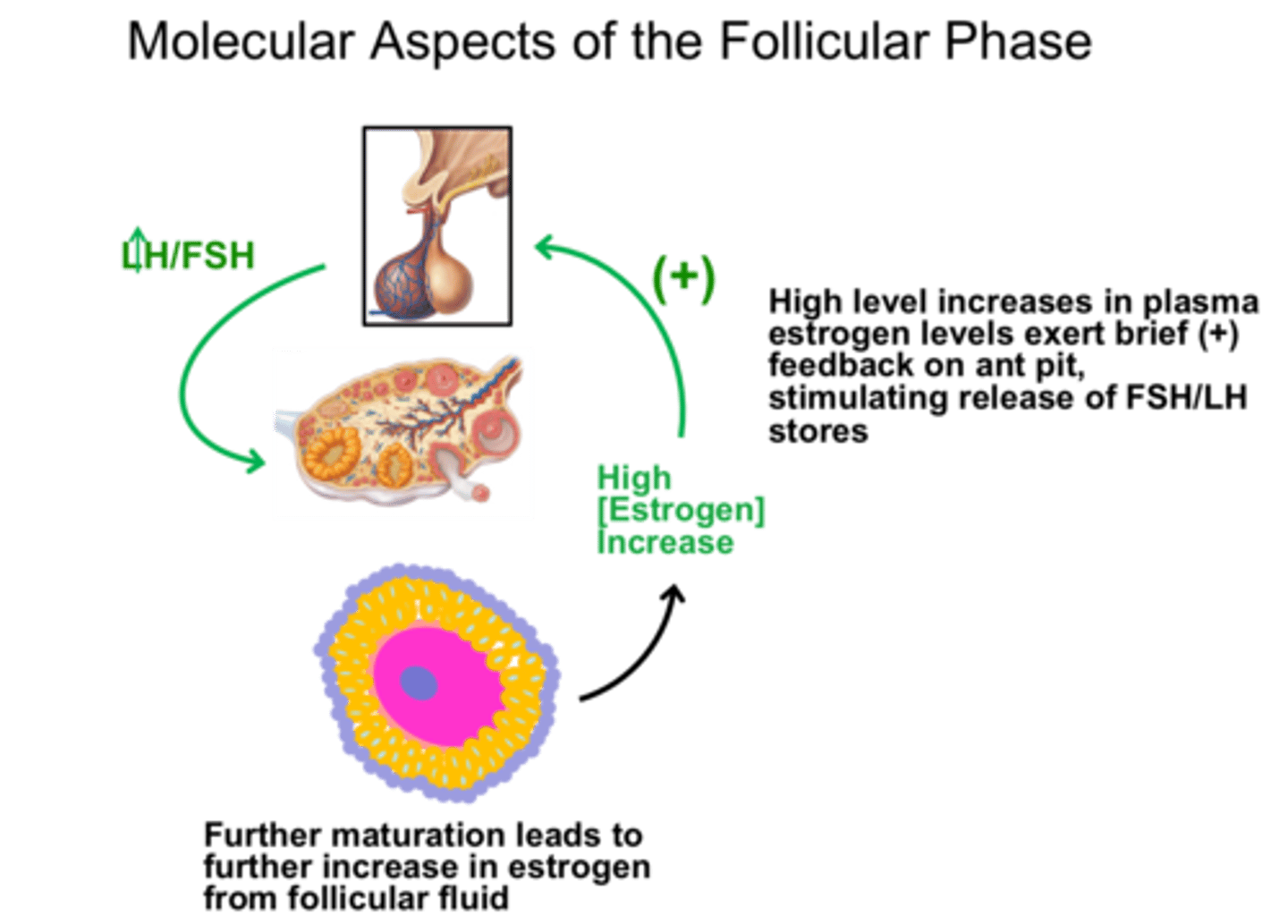
What are positive feedback loops
Response of the system goes in the same direction as the change that sets it in motion
Pituitary gland secretes LH that stimulates the ovaries to secrete estrogens, which regulate reproductive function
Under certain conditions, a rise in estrogen can trigger an increase in the secretion of LH which stimulates more estrogen which again stimulates LH (called "LH surge")
Some factor always acts to terminate the positive feedback, this case its ovulation
ie. labour (terminated by baby and release of placenta)
Signals of Homeostatic System
Hormonal (endocrine system)
Autonomic Nervous system (next week's lab)
- parasympathetic nervous system
- sympathetic nervous system
Homeostatic example: Girl pulled alive from rubble, 15 days after earthquake
found 15 days after, dehydrated, groaning weakly, but still conscious, with a very weak pulse and low blood pressure
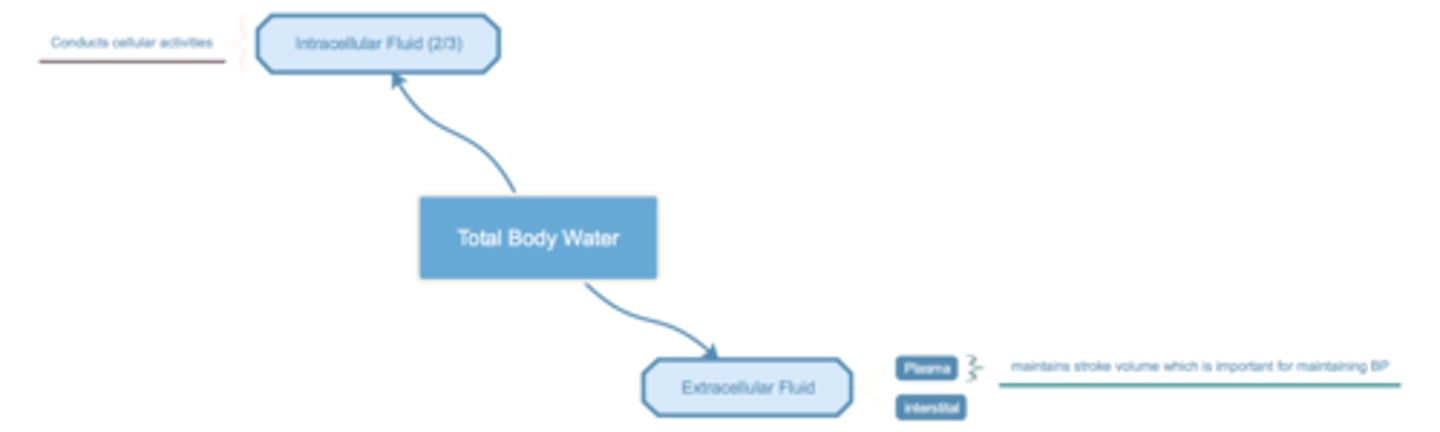
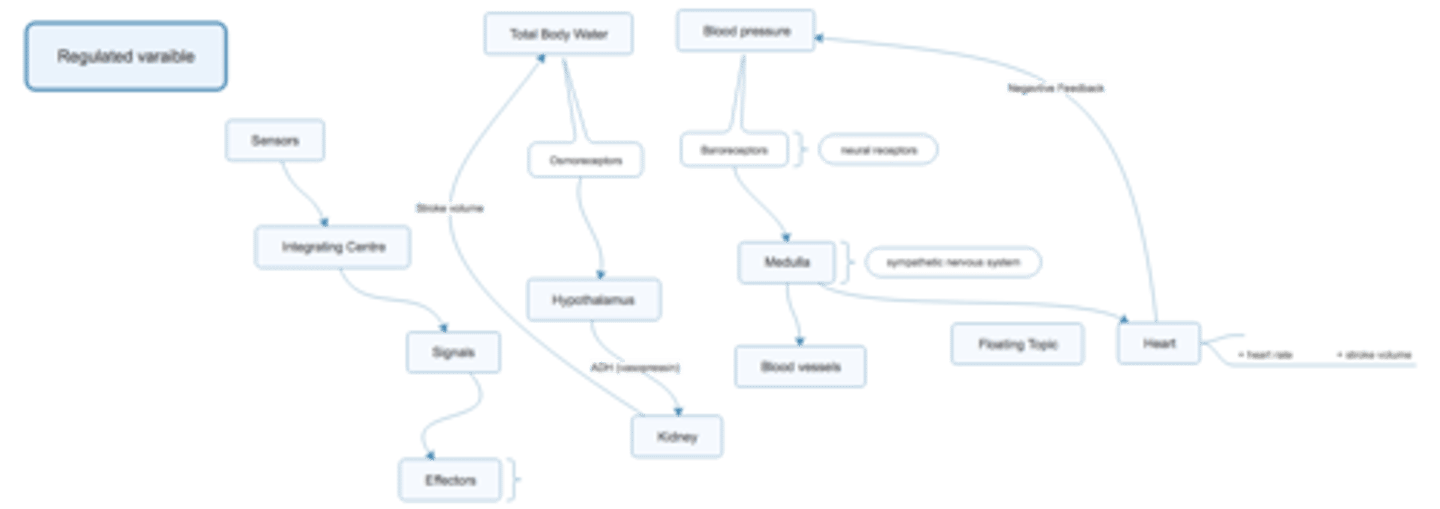
List the major waster compartments of the body and explain how water moves between them
TBW = total body water
150 lb, 42 L (60%) of water
ECF = extracellular fluid (1/3)
ICF = intracellular fluid (2/3 of TBW ~28 L)
ECF - plasma (3 L), interstital fluid (15 L?)
ICF - cell functions
plasma v. interstital
- plasma, maintains stroke volume which is important for maintaining BP (HRxSVxTPR)
****PICTURE
How would her body begun to lose water (earthquake example)
How to lose water:
- respiratory
- renal (urination)
- sweat
- gut (digestive processes)
How do you add water:
- drinking (N/A)
- eating (N/A)
- cellular metabolism (produced water + CO2)
- undergoeing starvation --> fat metabolism (instead of glucose) which produces more water
- reduction in respiration rate
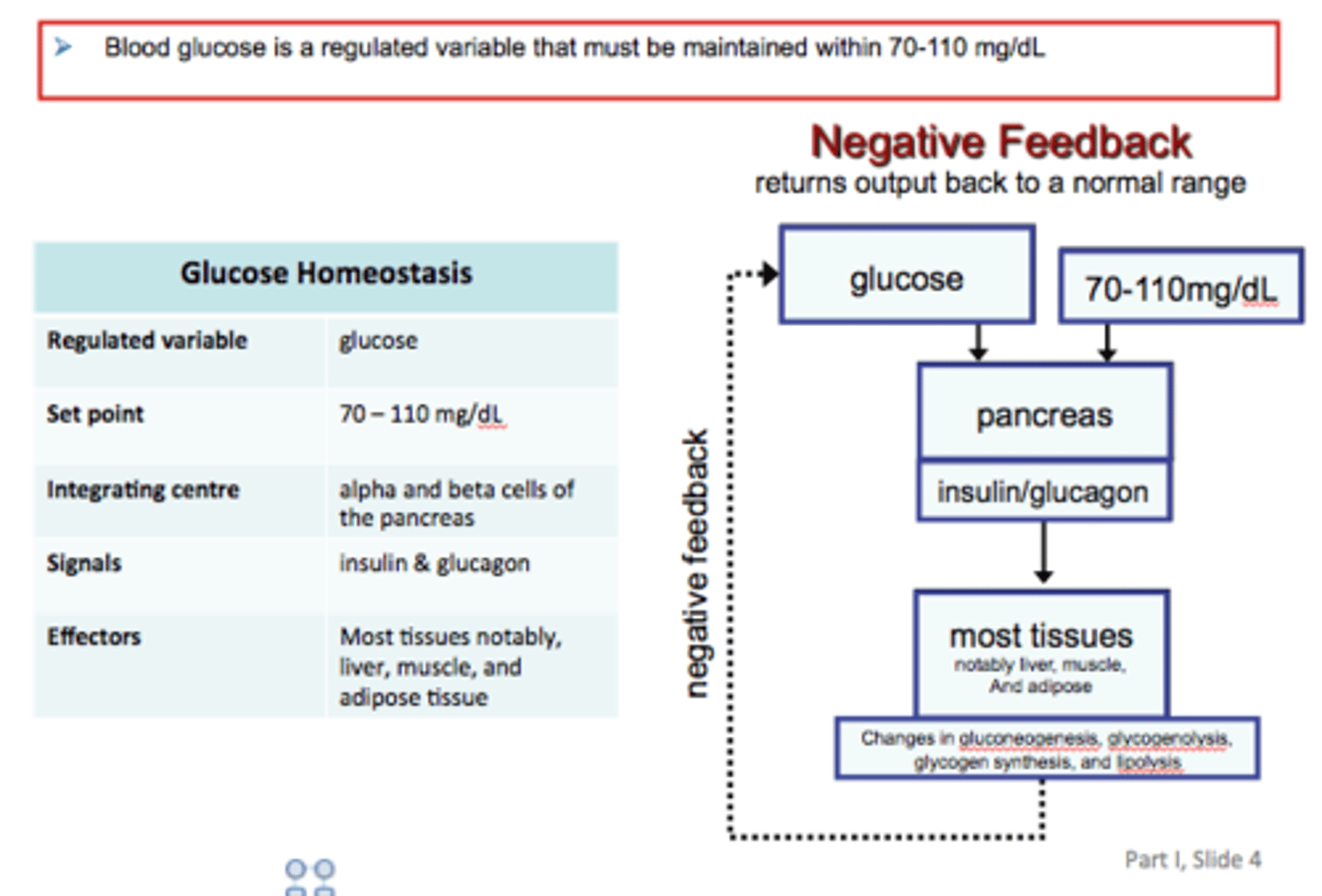
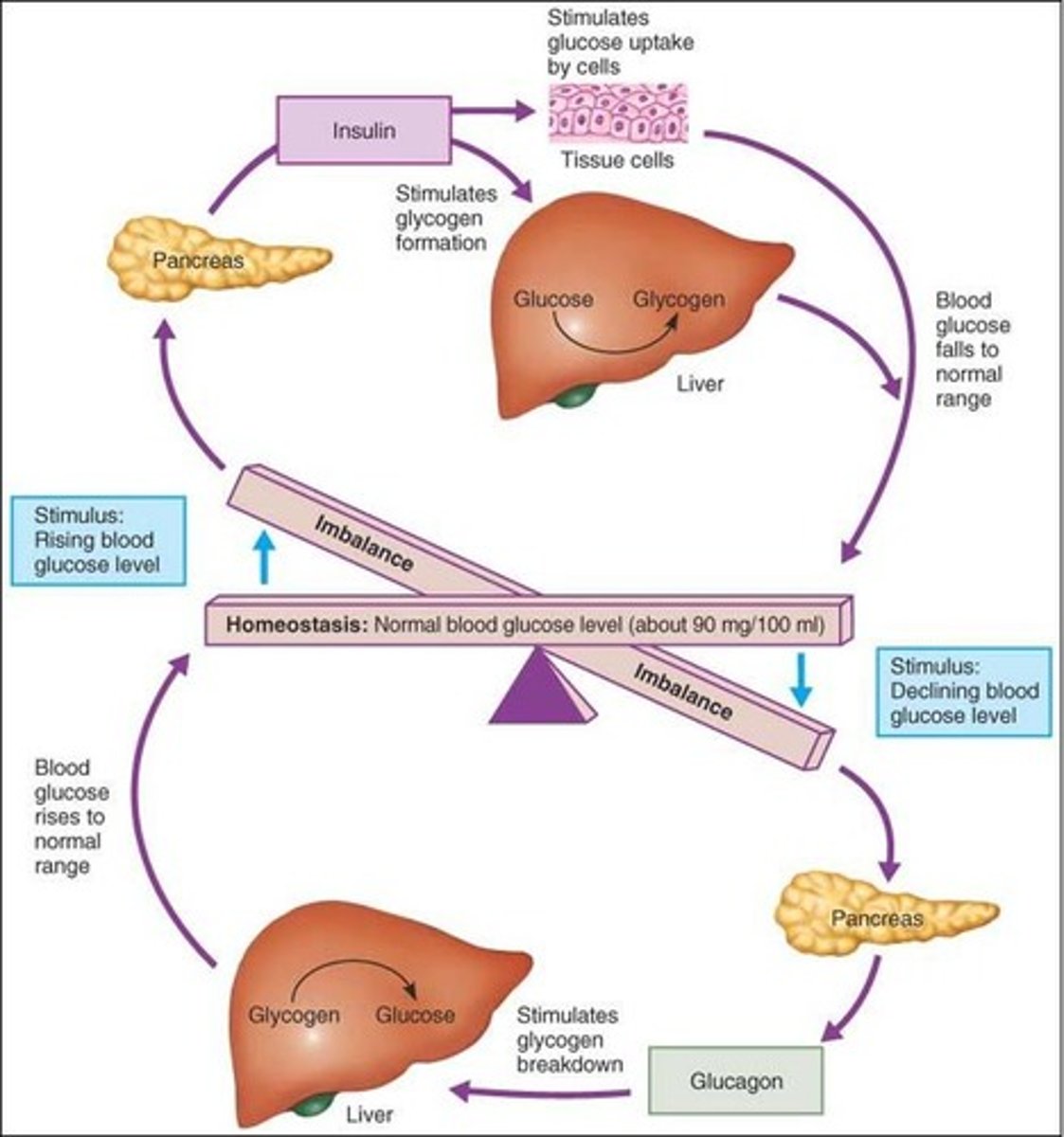
Glucose Homeostasis
1. Regulated variable: amount of glucose in blood
2. Set point: 100 mg/100 mL
3. Sensory: beta cells in pancreas
4. Integrating centre: pancreas
5. Effectors: liver (either decreases or increases glucose in the blood)
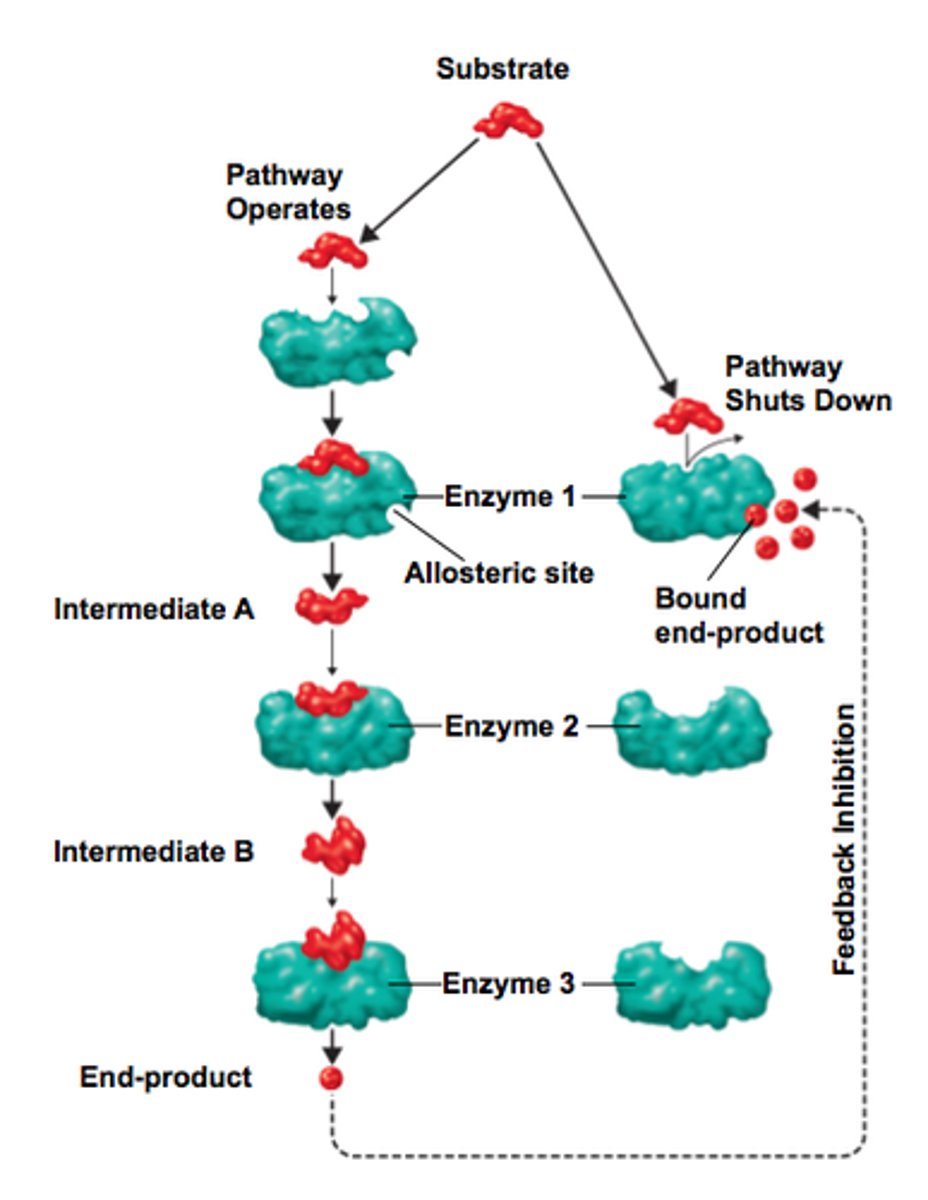
Feedback inhibition
Metabolic pathways have a site of regulation that corresponds to the rate limiting step
allosteric modulation: reversible
Enzyme has 2 sites, one for the modulator
ie. enzyme C (modulator) will acculumate and then will feedback to B (instead of all being used to form D)
Conformation change that is due to allosteric modulation
**Law of Mass Action
End product inhibition: feeds all the way back to the 1st enzyme
Feedforward Activation
when a metabolic product ACTIVATES an enzyme that catalyzes a reaction further of it in a metabolic pathway
ie. Glycolysis (fructose-1,6-biphosophate acts as an allosteric activator of pyruvate)
Law of mass action
- prime the entire pathway
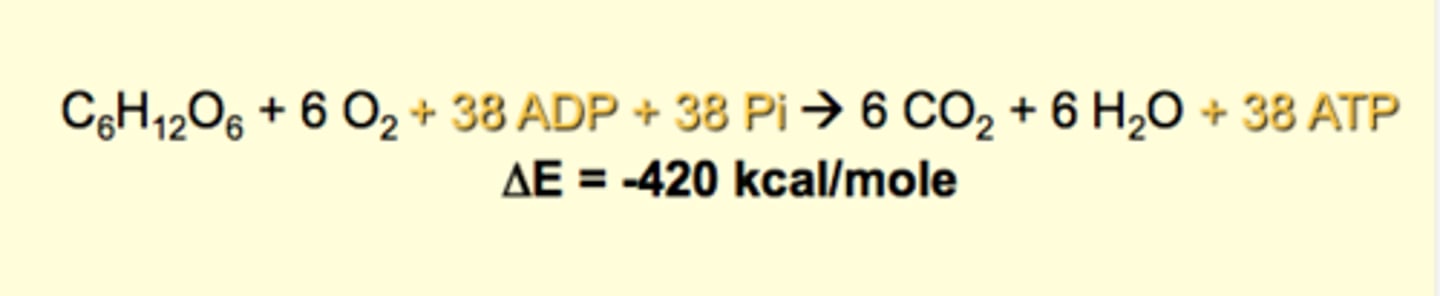
Glucose oxidation
Objective: cellular movement, molecular synthesis, transport accress membrane
- most energy is lost as heat
Case study
Symptoms:
- dizziness
- confusion
- headache
- shortness of breath/rapid breathing
- vomiting
most deaths were very rapid, occurring within a few hours of symptoms
1. are there are similarities or connections bettween these 7 individiuals?
2. what questions would you want to ask the families to hel solve this mystery?
3. in your opinion, are these 7 deaths connected? why or why not?
Glucose Oxidation: The Central Reaction of Energy Metabolism
- ATP hydrolysis (exergonic) ATP + (+H2O) --> ADP + Pi + energy : used for WORK
- ATP synthesis (condensation) ADP + Pi + energy --> ATP (+H2O)
ATP: The medium of energy exchange
ATP synthesis
- oxidative phosphorylation (ADP + Pi --> ATP)
- substrate-level phosphorylation (X-P + ADP --> ATP + X) creatine phosphat; allows us to quickly generate ATP for quick physical activity
Energy Exchange in Glucose Oxidation
- major production of heat
- occurs spontaneous (neg.) v. ATP which is not (pos.)
- only 33% of the energy from glucose oxidation is captured and stored as ATP
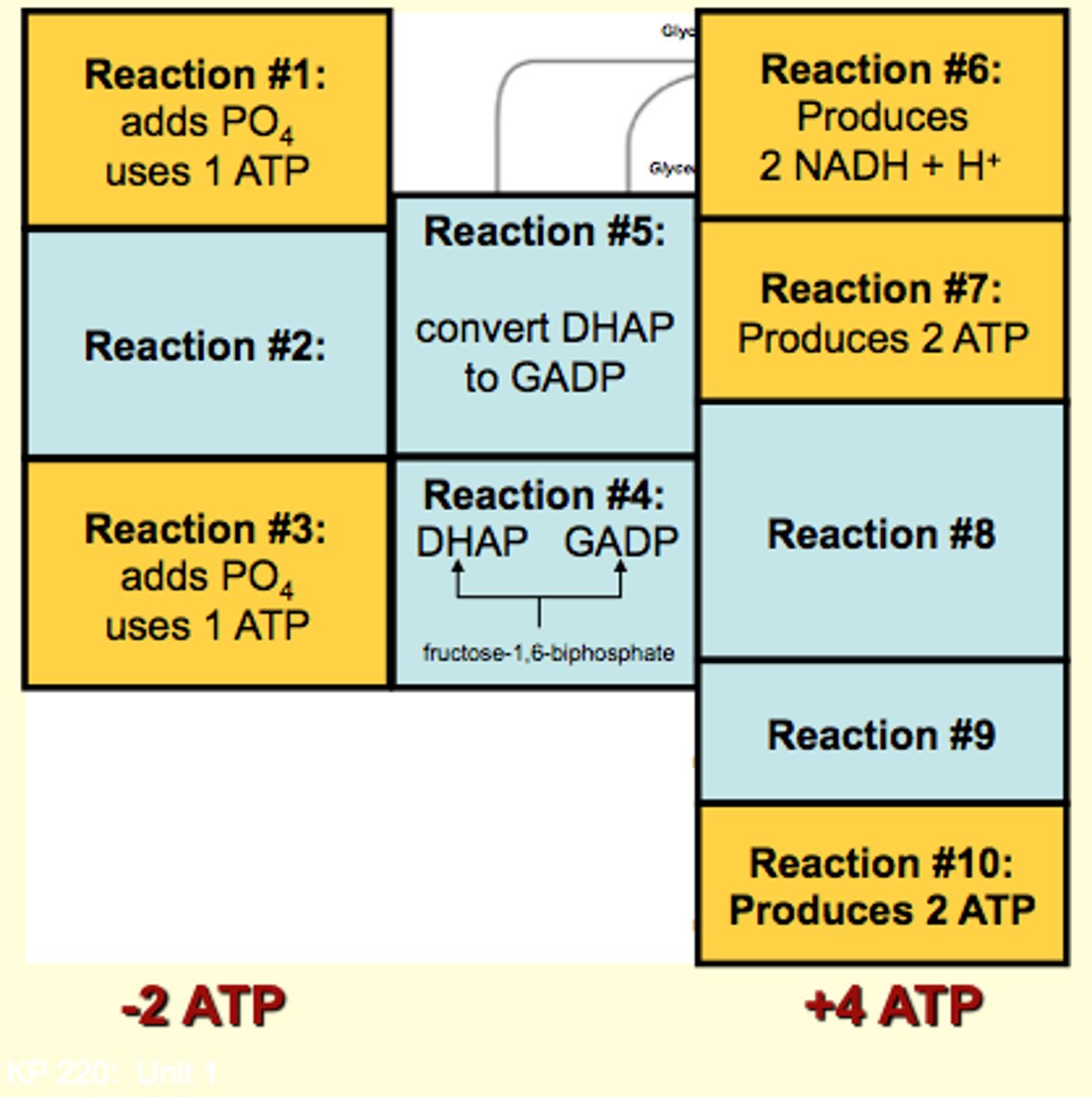
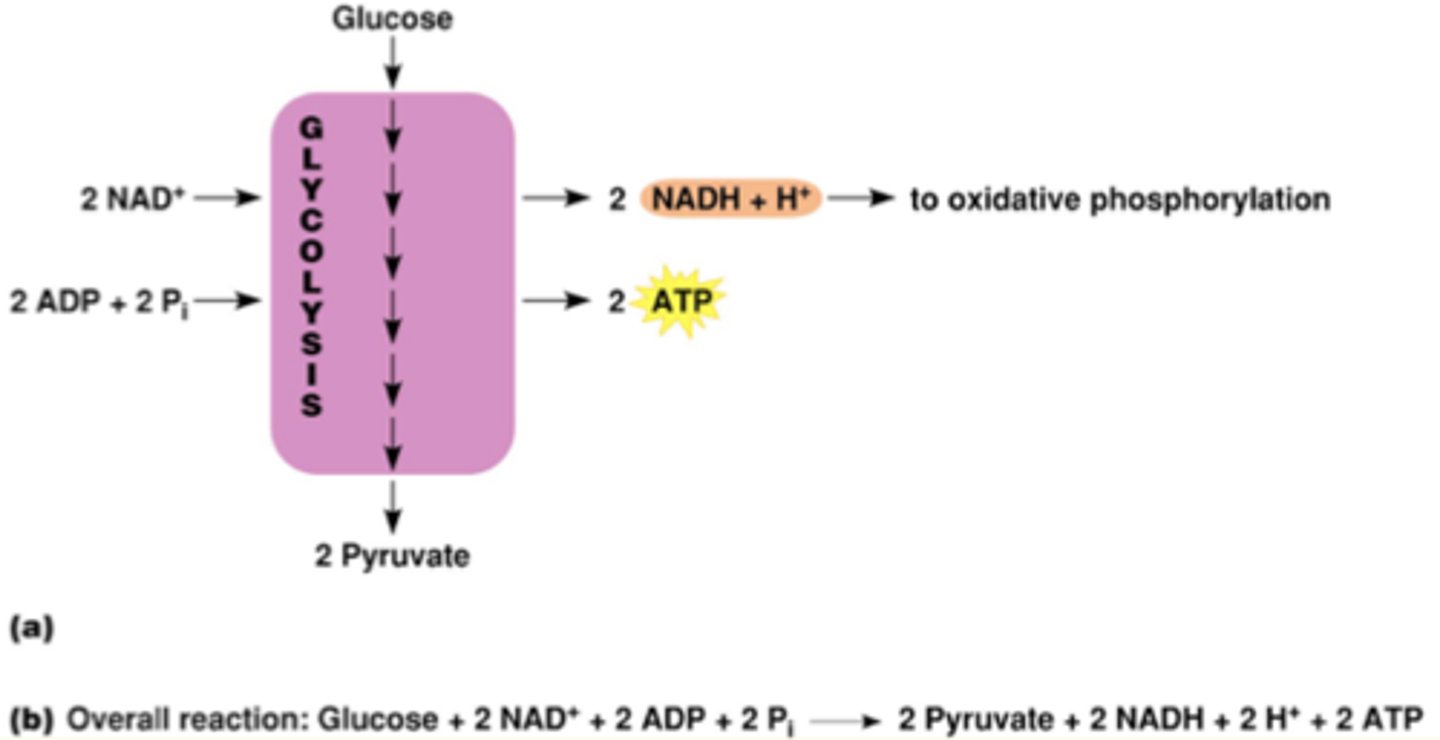
Glycolysis Summary
1. each glucose will split into 2 pyruvates
2. 2 ATP molecules are consumed (rxn 1 and 3), however 4 are produced by substrate-level phosphorylation; net gain of 2 ATPs
3. 2 molecules of NAD+ are reduced in step 6; 2 molcules of NADH per glucose
- glucose-oxidation occurs IN cytosol
- no oxygen consumed/no CO2 produced in glycolysis but does occur in glucose-oxidation
1st half of glycolysis: Uses 2 ATP
2nd half of glycolysis: Produces 4 ATP
Net production: 2 ATP
Glycolysis feedback inhibition example
Glucose-6-phosphate (product of 1st reaction) will feedback to hexokinase, to inhibit
Feedback activation: Fructose-1,6-biphosphate will feedback pyruvate kinase
Glyosis (Step 4)
1 3-Carbon sugars to 3 3-Carbon sugar
Glyolysis Step 6
- produces reduced coenzymes
- used later to produce ATP through oxidation-phosphorylation
Glyolysis Step 7-10
Actually produces ATP
(2 of everything)
What is the purpose of Glycolysis?
Purpose: it sets up the stage for subsequent events that yield even more ATP
ie. the NADG it produces will give up its electrons, release energy that will be used to produce more ATP and the pyruvate produced will be catabolized
Kreb's cycle definition
Cyclical metabolic pathway that uses acetyl CoA as the initial substrate and reduces coenzymes
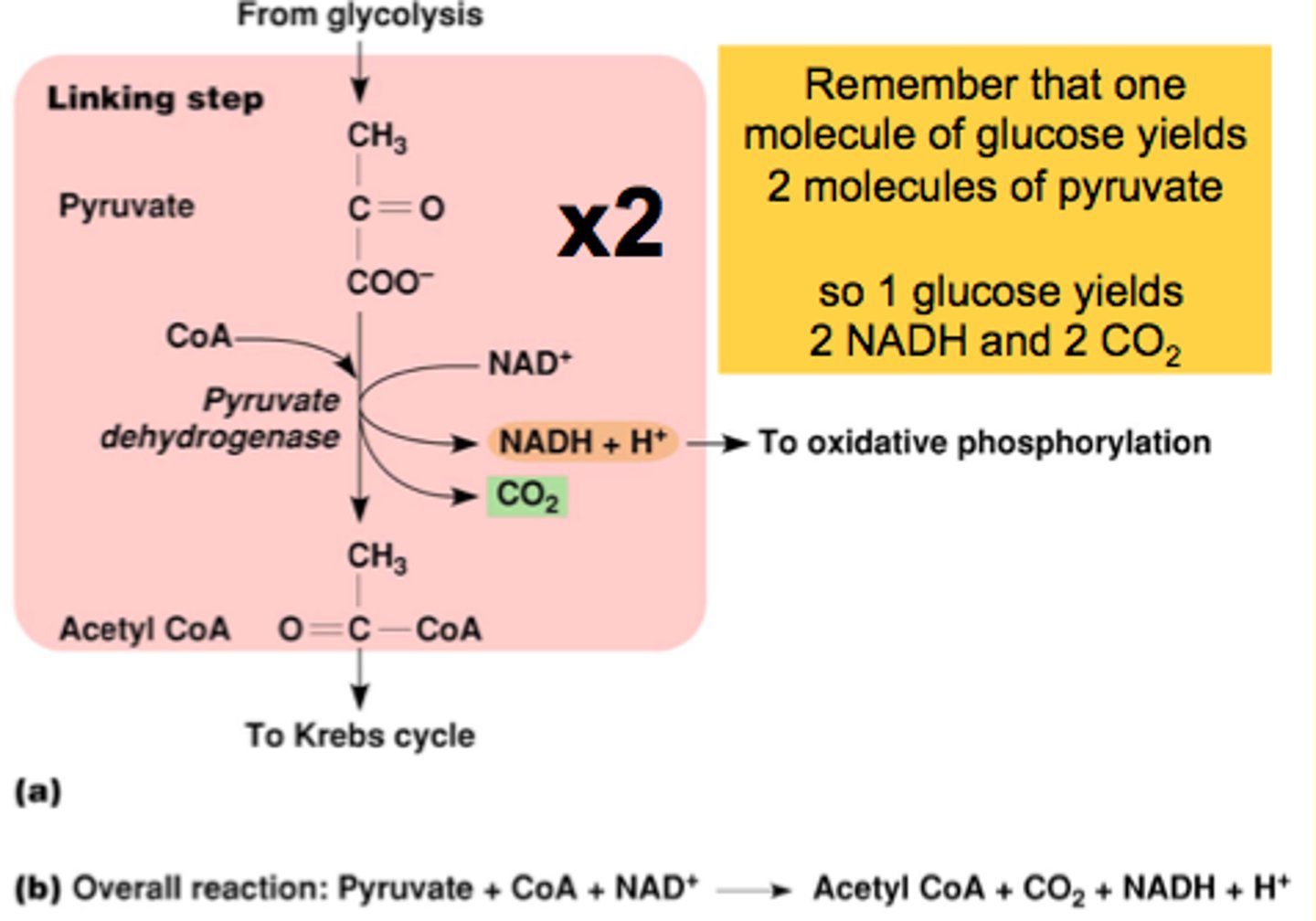
What is Glucose-Oxidation?
It is the linking step between Glycolysis and the Krebb's cycle
Starts with 2 pyruvates forming (in cytosol)
Pyruvate is converted into acetyl CoA in the mitochondrial matrix (by NAD+ reducing to NADH + H+ and CO2)`- 2 of each bc there is 2 molecules of pyruvate
Kreb's Cycle Reaction 3 and 4
Produces CO2
- reduced coenzyme (NADH) + H+
Kreb's Cycle Reaction 6
- different type of reduced coenzyme
Kreb's Cycle Reaction 7
- original reduced coenzyme
- NO CO2
Reactions of the Kreb's cycle
**2 turns of the cycle per glucose molecule
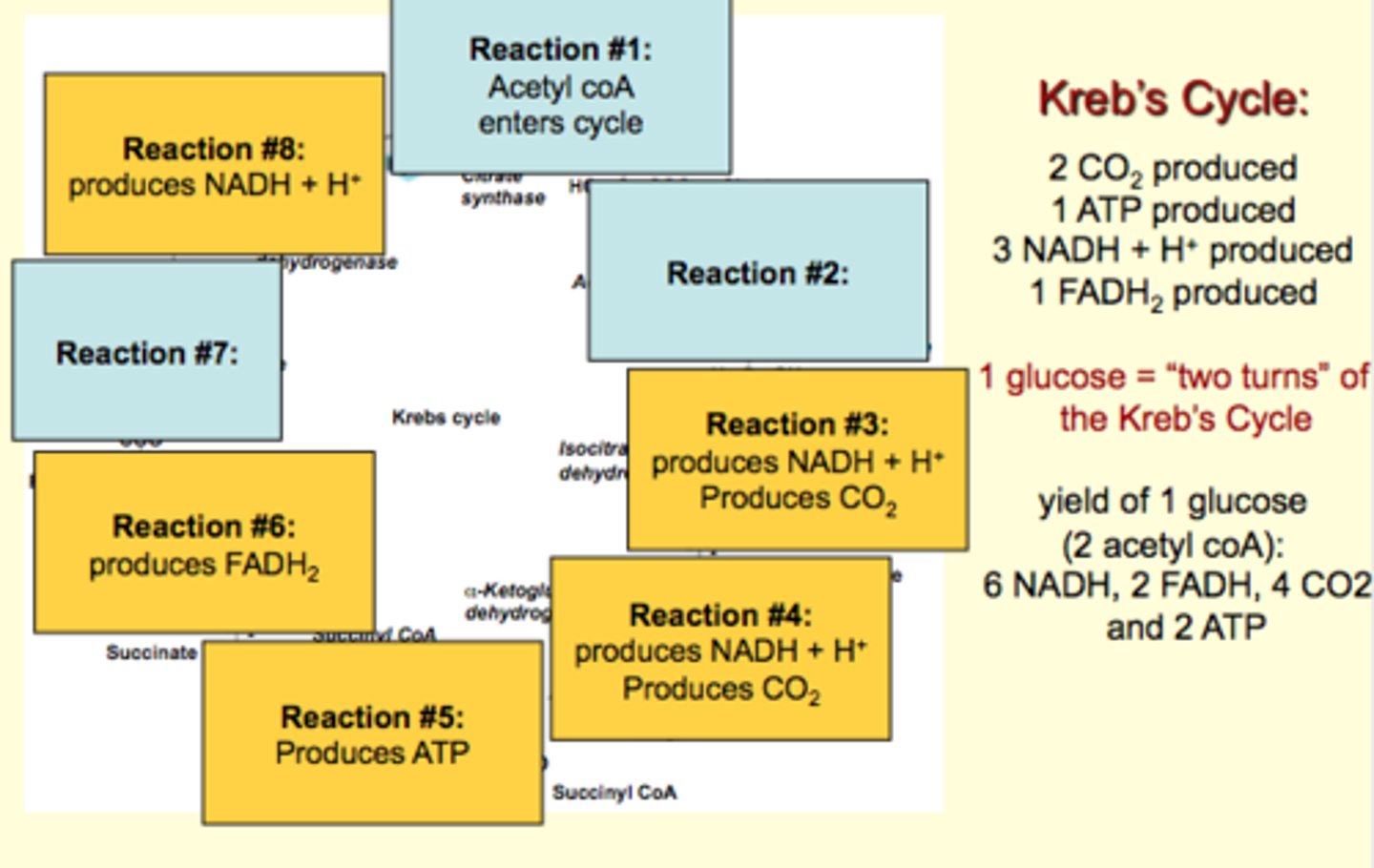
Summary of the Kreb's Cycle
Remember that one molecule of glucose yields 2 molecules of pyruvate (converted to 2 molecules of acetyl coA in linking step)
therefore
1 glucose = "two turns" of the Kreb's Cycle
Resulting in 6 NADH, 2 FADH, 4 CO2 and 2 ATP
Final product: oxaloacetate + acetyl CoA starts the cycle over again
Net: 4 ATP per molecule of glucose
The Mystery of the Seven Deaths
Part II - Autopsy Report
Immediate cause of death was hypoxia (suffocation or lack of oxygen).
Tissue sections from heart, lung, kidney, and liver all show massive cell death.
Staining with specific dyes showed major mitochondrial damage within the affected tissues.
Oxygen levels in the patients' blood were approximately 110 mm Hg (normal range is 75 - 100 mm Hg).
***died of hypoxia yet there was high levels of oxygen
What is Oxidative Phosphorylation?
Use of movement of electrons down the electron transport chain to synthesize ATP
- requires ETC in mitochondria and oxygen
- most ATP in cells in made from this
2 Process:
1. Electron Transport Chain (inner mitochondrial membrane) uses NADH+H+ and FADH2
2. Chemiosmotic coupling (harnessing of energy released from ETC)

How the Electron Transport Chain works?
Occurs in the mitochondrial membrane, with specialised proteins (compounds) that function as electron carriers that pick up and give up electrons
- Molecules undergo oxidation/reduction reactions from high to low energy
- Energy released used to synthesize ATP by oxidative phosphorylation
- Last electron acceptor = O2
**matrix = inbetween 2 membranes
FADH in Electron Transport Chain
- enters Kreb through Coenzyme Q
NADH in the Electron Tranport Chain
enters Kreb through Cytochrome C
- approximately 1.5 ATP molecules per pair of electrons released
ATP Production per Electron Donor
- depends on efficiency of Electron Transport Chain
- range of efficiency

The Mystery of the Seven Deaths - Part III
build up reactants and reduction in products of oxidative-phosphorylation and therefore a failure of Electron Transport Chain
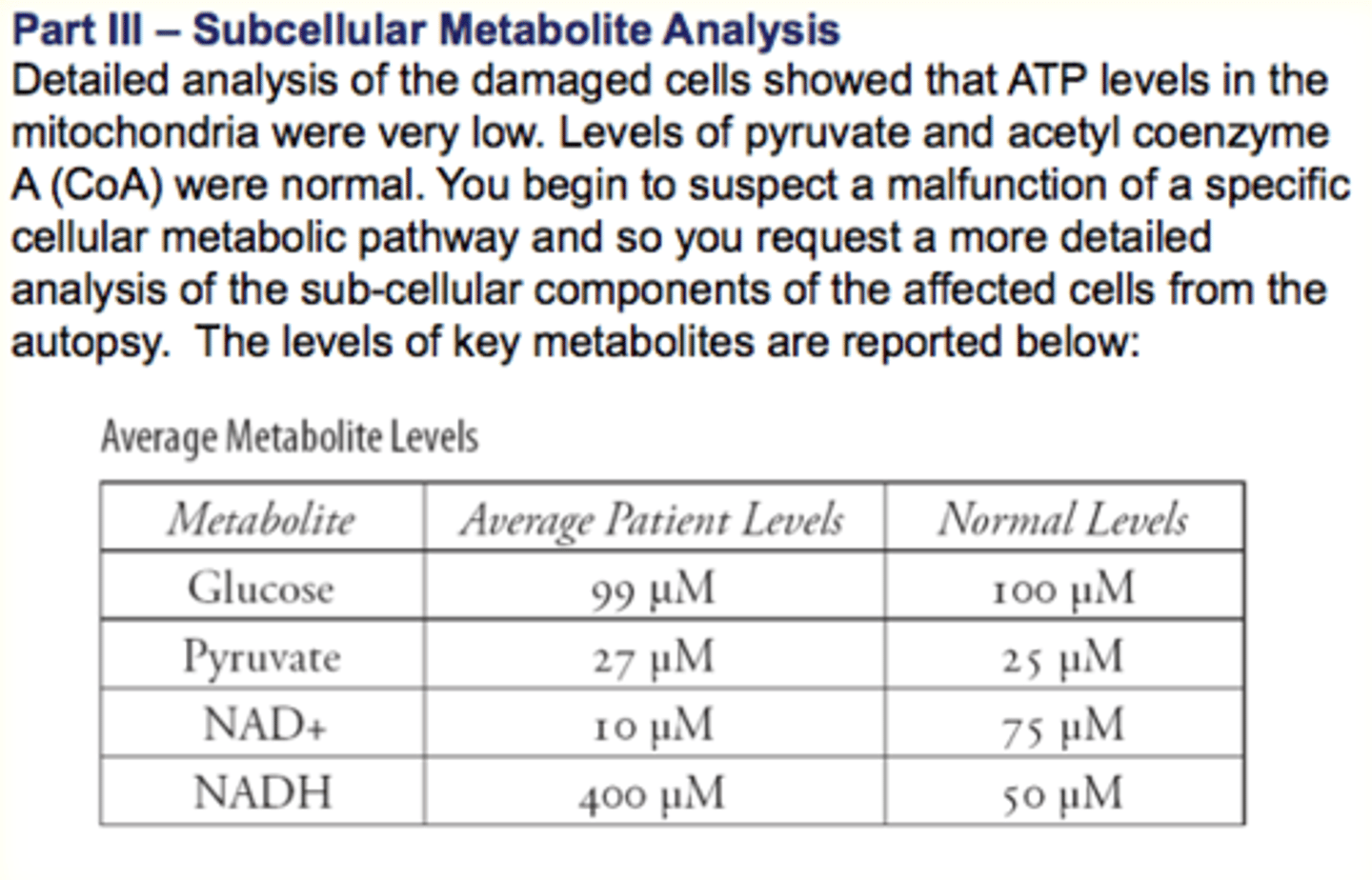
Chemiosmotic Coupling
Coezneyme Q ad Cytochrome C can both travel through the membrane
Energy released (from oxidation-reduction reactions as electrons travel through complexes) and used to move H+ ions against their concentration gradient and into the intermembrane space
Concentration gradient is used to fuel ATP synthase
- phosphorylate ADP into ATP
- synthase utilizes the energy released from the concentration gradient to make ATP from ADP
Reason for ranges in ATP production
leaking mitochondiral memebrane = system becomes less efficient and gradient
Mystery: Answer
- all patients were exposed to cyanide
- ETC no longer functioning
- cyanide binds to cytochrome C oxidase irreversibly
- CcOX of the ETC prevents the trans of electrions to oxygen, the final electron acceptor
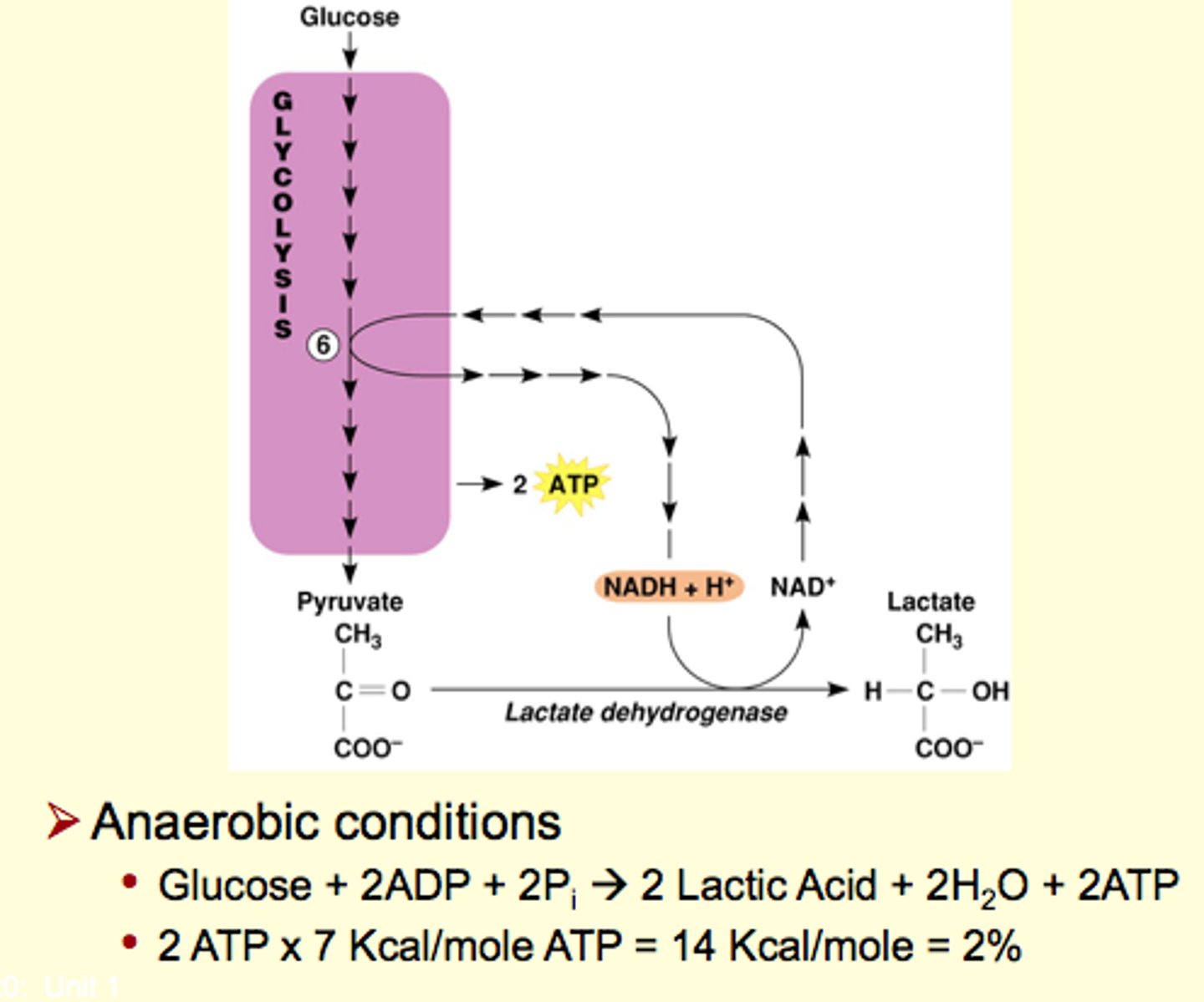
Glucose Catabolism in Absence of Oxygen
Low oxygen availability
- Electron transport in chain backs up
- Krebs cycle stops
Glycolysis can continue only if NADH is oxidized
Step 6
requires an oxidized coenzyme (under low oxygen environment)
alternative method: convert pyruvate into lactic acid
- not efficient
- used if ETC is not working at the moment
lactate shuttle is used to at least maintain 2 ATP per glucose
Energy Storage and Use
Can use fats and proteins as alternative fuels
must be broken down before entering glucose oxidation pathway
- Can also store energy as fat, protein or glycogen
**dont need to study protein or fat metabolism
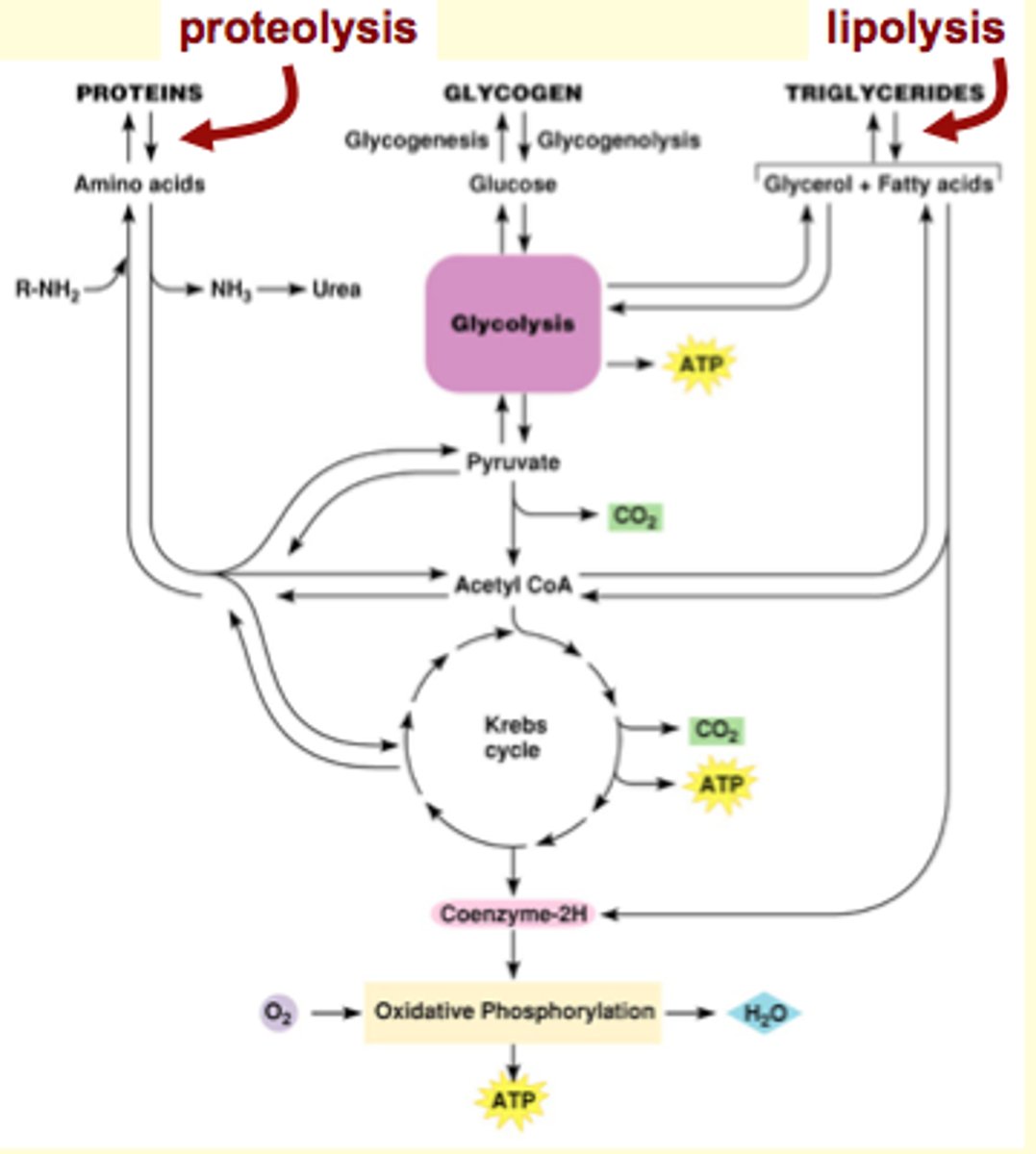
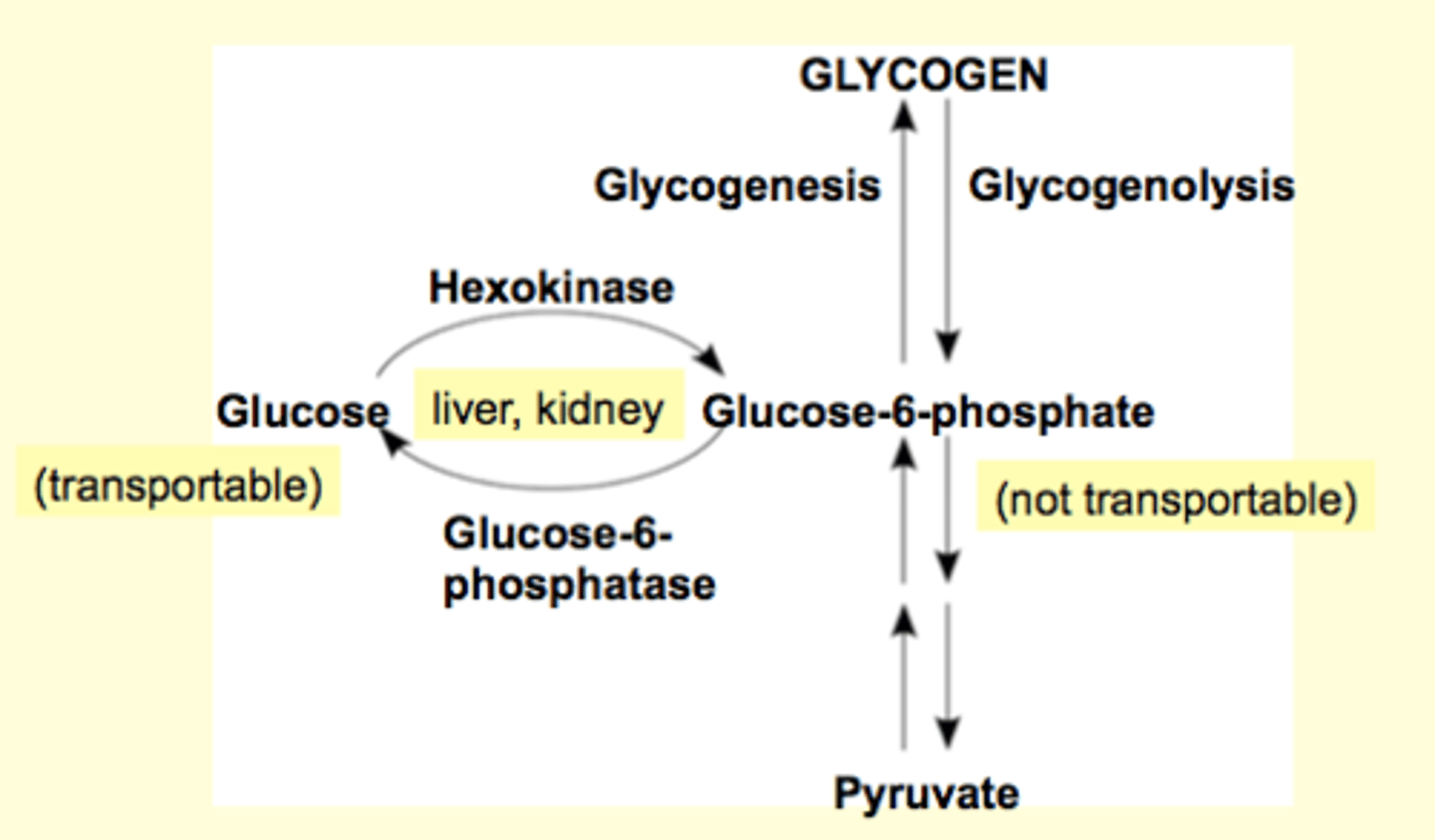
Glycogen Metabolism
Skeletal muscles and liver are very good at glycogenesis
Liver (and kidney, a little bit) glycogen stores provide glucose for other cells (via blood). Other tissue cannot.
liver and kidney have glucose-6-phosphatase (enzyme), which works backwards to make glucose from glucose-6-phosphate and release it into the blood stream to share it (glucose) with other tissues
Extracellular fluid
- interstitial fluid, plasma
- have different concentrations of
What's more concentrated inside/outside of cell?
K inside, Na outside
- lipo fatty acid can pass through phospholipid bilayer easily
water cannot as it is lipo phophobic (or something)
ie. needs endo/exocytosis, active transport, facillated diffsion
Objectives/Questions

Concept Map
Draw a concept map using the following terms:
membrane transport
membrane permeable
membrane impermeable
no energy input required
energy input required
channels
transporter
simple diffusion
osmosis
facilitated diffusion
primary active transport
secondary active transport
What are the forces that drive the movement of substances across a membrane?
1. Chemical driving force
2. Electrical driving force
3. Equilibrium potential
Chemical driving force
- Concentration gradient = ∆C
- tendency of particles to move from higher to lower concentration
- Force acts from higher to lower concentration
- Rate depends on magnitude of ∆C
*Energy of solution (Gi and Go)
Free energy of solution:
Go ---> G = RT ln[S]
![<p>- Concentration gradient = ∆C</p><p>- tendency of particles to move from higher to lower concentration</p><p>- Force acts from higher to lower concentration</p><p>- Rate depends on magnitude of ∆C</p><p>*Energy of solution (Gi and Go)</p><p>Free energy of solution:</p><p>Go ---> G = RT ln[S]</p>](https://knowt-user-attachments.s3.amazonaws.com/55928393-6449-441f-869a-c7d0564c2da5.jpg)
Energy of Solution
free energy of solution
G = RT ln[S]
∆G = Gi - Go
∆G = RT ln [S]i - RT ln [S]o
when the outside con. is greater, the denominator is bigger than the numeorator (a FRACTION)
a ln <1 is gonna me a negative number
the difference between the two energy is negative, and therefore it does not require energy and will move spontaneously to the lower energy environment
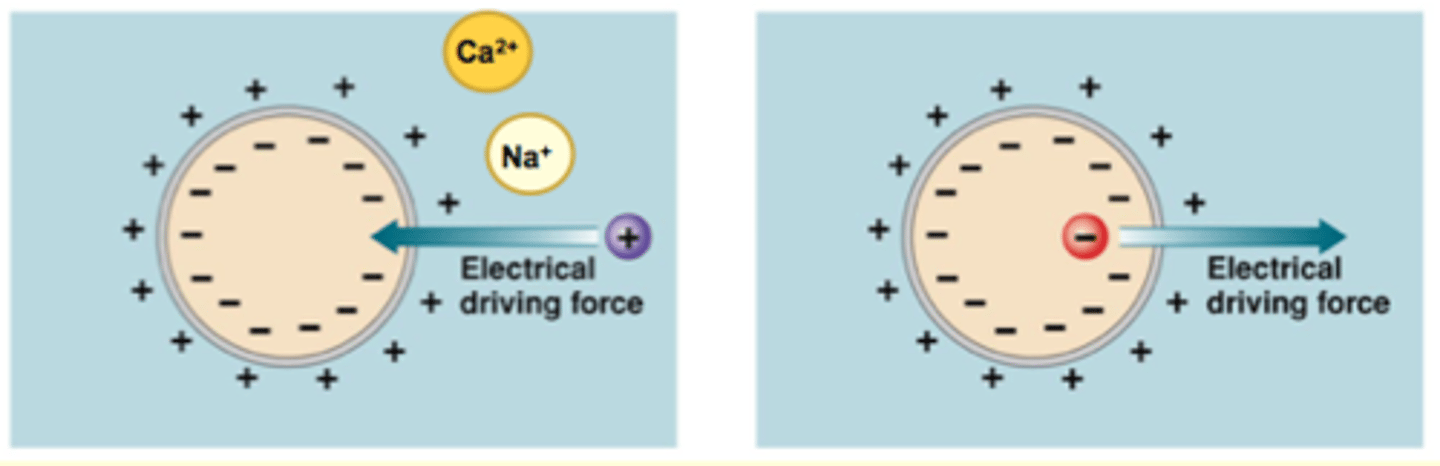
What are the 2 things that affect electricial driving force?
1. Charge across membrane (Vm) or membrane potential
2. Charge of the solute
how these 2 factors interact: "like repels like and opposites attract"
**always talk about the inside of the cell compared to the inside
Electrical driving force
Membrane potential (Vm)
- Due to unequal distribution of anions and cations across cell membrane
- Charge separation = source of energy
Magnitude of force depends on
- Strength of Vm
- Amount of charge on particle
- +/- charge refers to inside the cell relative to the outside
- opposites attract, like repels like; ions being "pushed" in one direction
**Ca 2+ has a larger magnitude and will be even more attracted to a negative environment
Electrochemical Driving Force
Negative charge inside, but K+ is positive inside cell
**is synonymous with chemical driving force in the case of uncharged particles
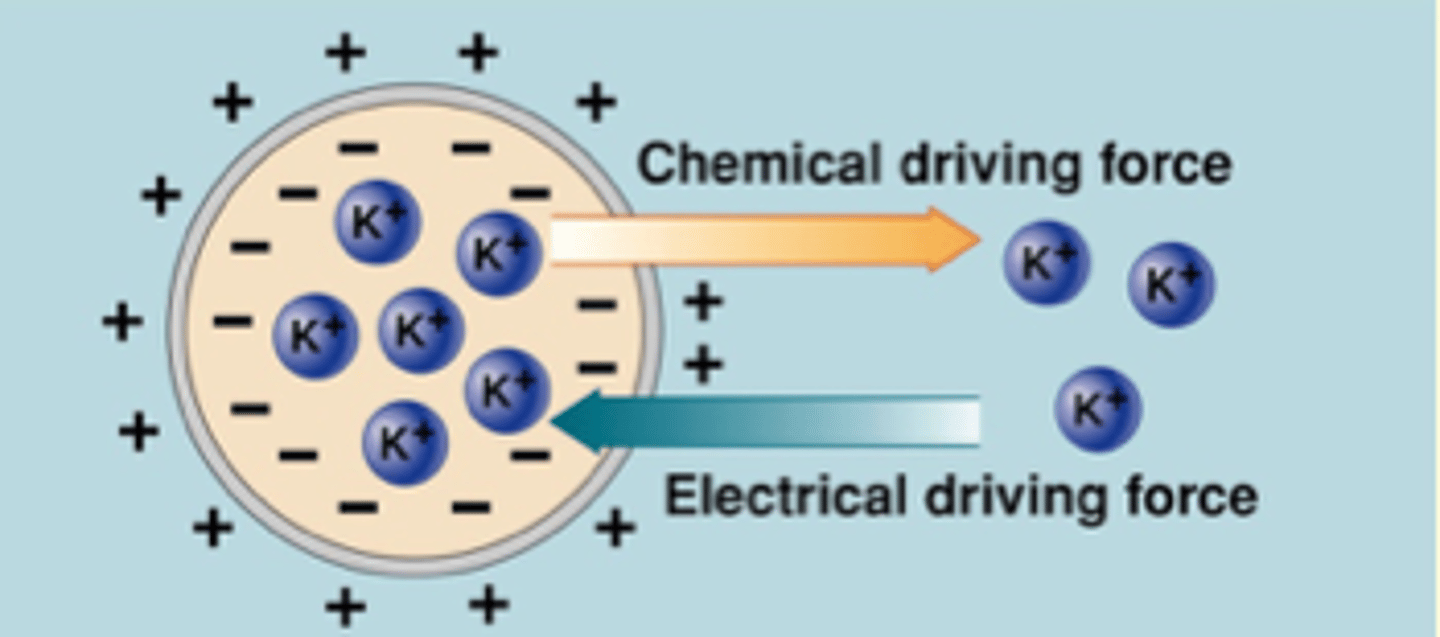
What is the Equilibrium Potential
What is membrane potential?
At rest - resting membrane potential (different cells have different)
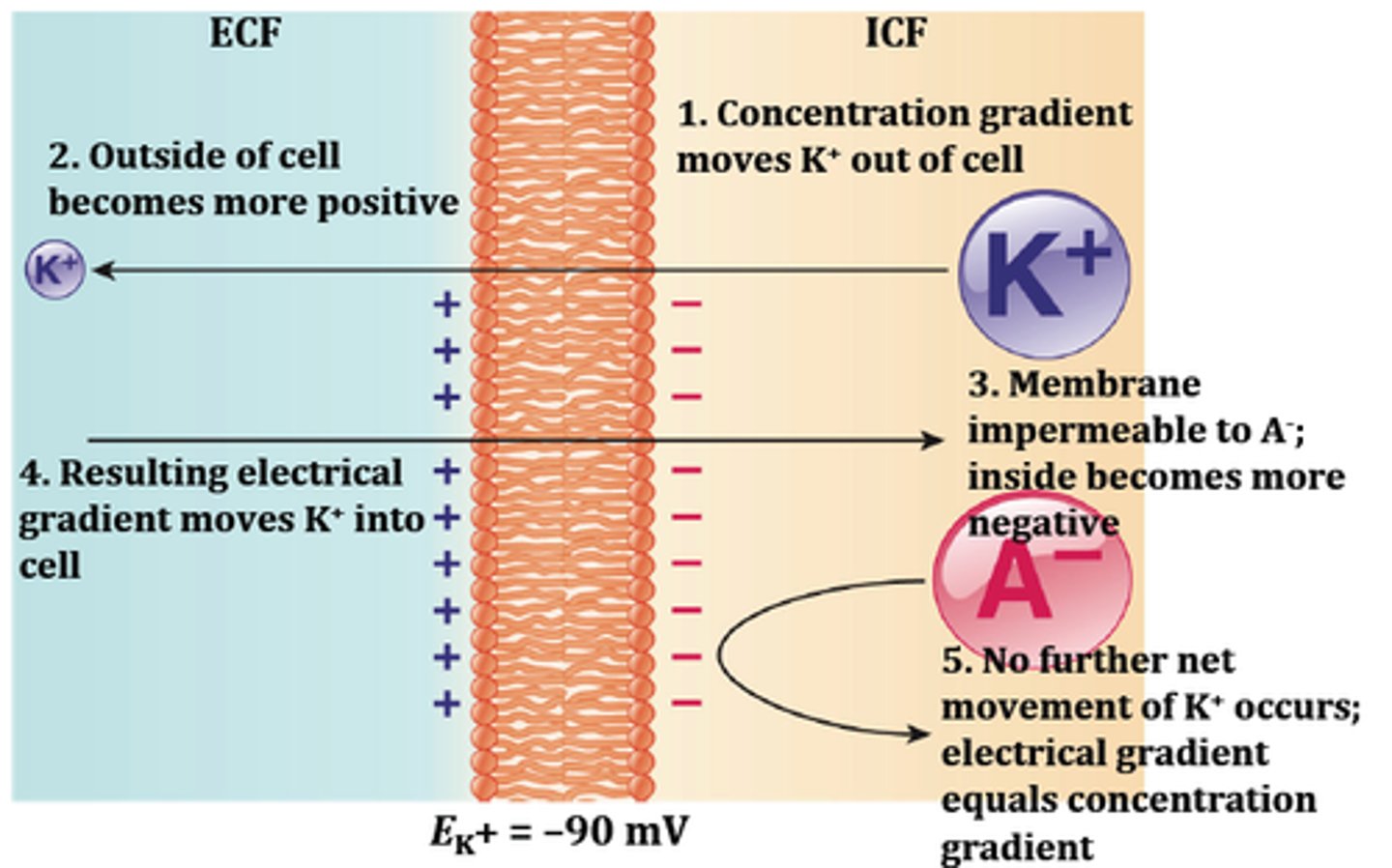
Each ion has an equilibrium potential (E ion)
The equilibrium potential is the voltage across the membrane that balances the chemical driving force
At equilibrium potential, the electrical driving force = to the chemical driving force
What is the definition of equilibrium potential?
voltage across membrane that balances the chemical driving force
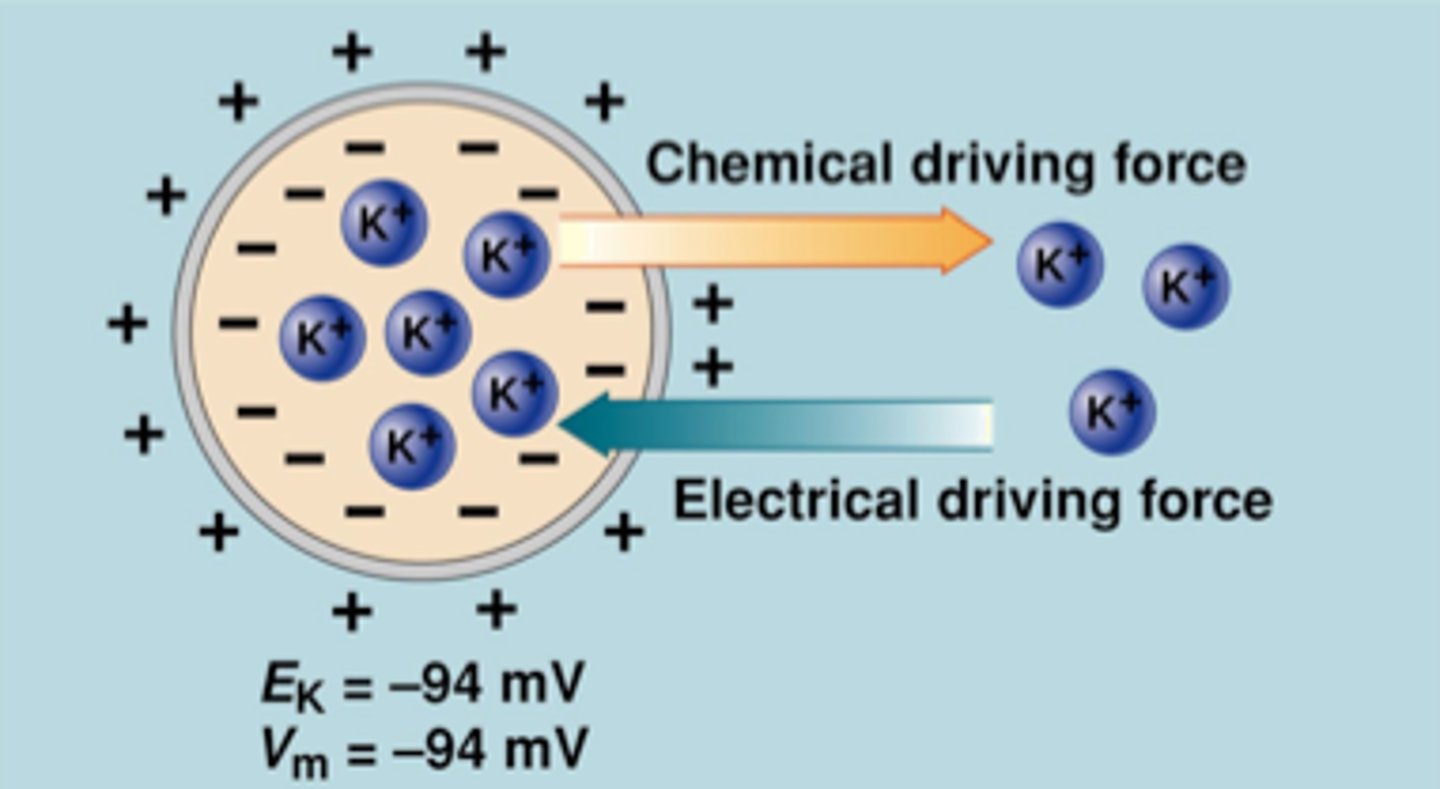
Ek = -94 mV
Vm = -94 mV
^ balancing
**equal but opposite arrows = chemical / equilibrium potential
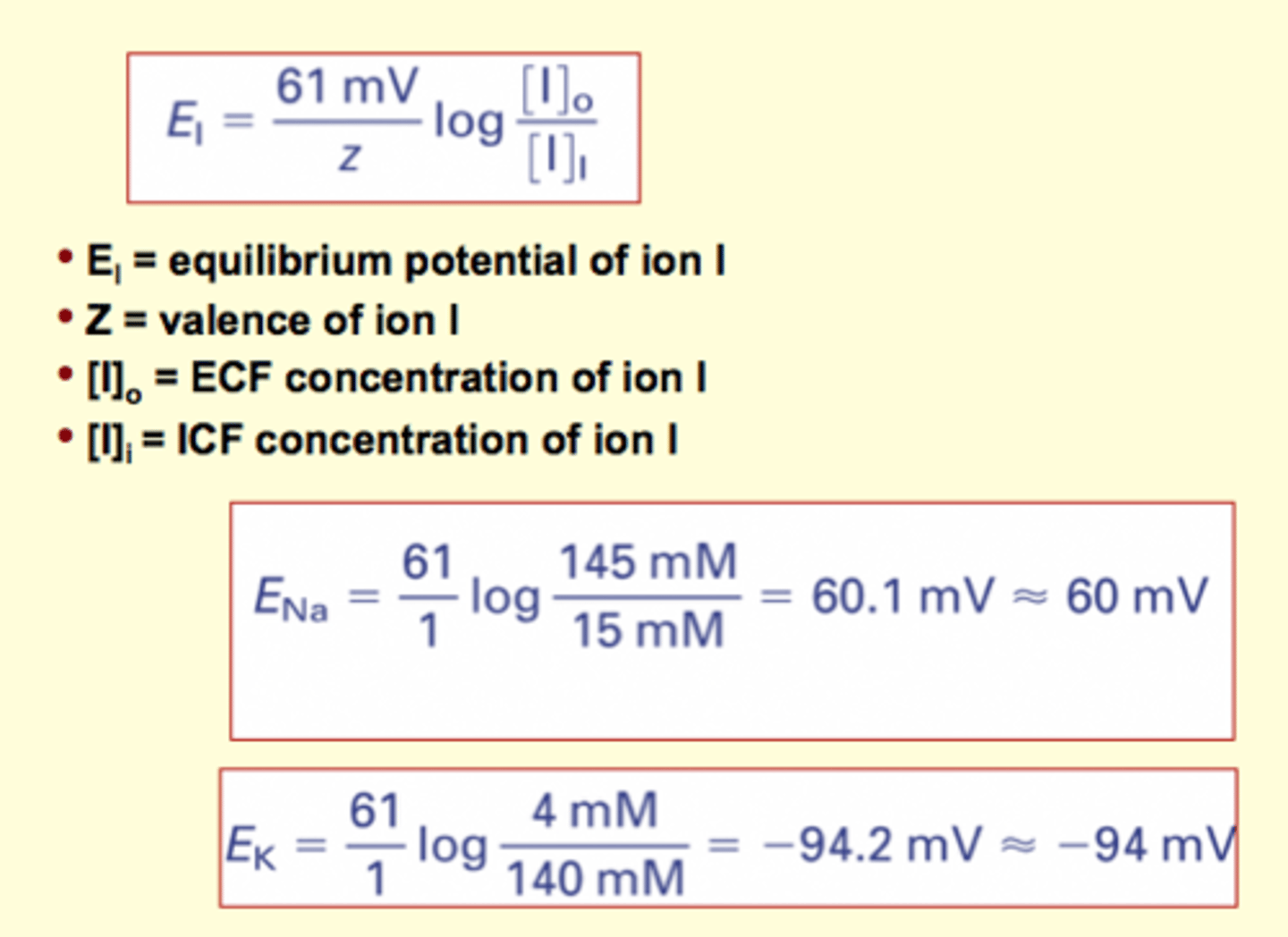
Calculating the equilibrium potential of an ion
El = equilibrium potential of ion l
Z = valence of ion l
[I]o = ECF concentration of ion l
[I]i = ICF concentration of ion l
T = absolute temperature (K)
R = universal gas constant (0.082 l·atm/mole·K)
F = Faraday's constant (9.54x104 joules/volt·mole)
picture: sets delta G = 0 (@ equilibrium)
solve charge across membrane that makes delta G = 0 (ie. no net flux, electrical and chemical driving force are equal and opposite)
Nernst equation: El = (61 mV /z ) log ([I]o/[I]l]
![<p>El = equilibrium potential of ion l</p><p>Z = valence of ion l</p><p>[I]o = ECF concentration of ion l</p><p>[I]i = ICF concentration of ion l</p><p>T = absolute temperature (K)</p><p>R = universal gas constant (0.082 l·atm/mole·K)</p><p>F = Faraday's constant (9.54x104 joules/volt·mole)</p><p>picture: sets delta G = 0 (@ equilibrium)</p><p>solve charge across membrane that makes delta G = 0 (ie. no net flux, electrical and chemical driving force are equal and opposite)</p><p>Nernst equation: El = (61 mV /z ) log ([I]o/[I]l]</p>](https://knowt-user-attachments.s3.amazonaws.com/58b09c3a-c3e2-4176-a6db-037eb5c26b7a.png)
Body temp in Kelvin
310.15 K
THE NERNST EQUATION
ie. Na = equilibrium is +60 (relative to the outside of the cell)
chemical driving force = to inside of cell (since Na+ is more concentrated outside the cell)
therefore, +60 (electrical driving) going outside of the cell
K+: more concentrated inside cell
Needs an electrical driving force (large, -94 mV) going inside cell
Direction of the Electrochemical Driving Force
To solve a lot of problems:
1. If Ei > Vm then chemical driving force "wins" (ion goes in direction of chemical)
2. If Ei < Vm then the electrical driving force "wins" (ion goes in direction of electrical)
Explanation of Direction of the Electrochemical Driving Force
An ion would "like" to move in the direction that will bring the membrane to its equilibrium potential.
Therefore we can determine the direction of movement using the following steps:
Identify the chemical and electrical driving forces. If they go in the same direction...you're done!
If the electrical and chemical driving forces act in different directions, compare magnitude of equilibrium potential and the membrane potential.
Equilibrium Potential (Ex)
Ek = Vm
Equilibrium Potential Problem
(b) Ei > Vm
Therefore, to bring the cell to equilibrium potential, you need to balance electrical driving force and you can do that by ion moving in direction of chemical driving force
(c) Ei < Vm
**can only move positive charge, therefore
ion moves in direction of electrical driving force if Electrical driving force is going outside then it is the same charge
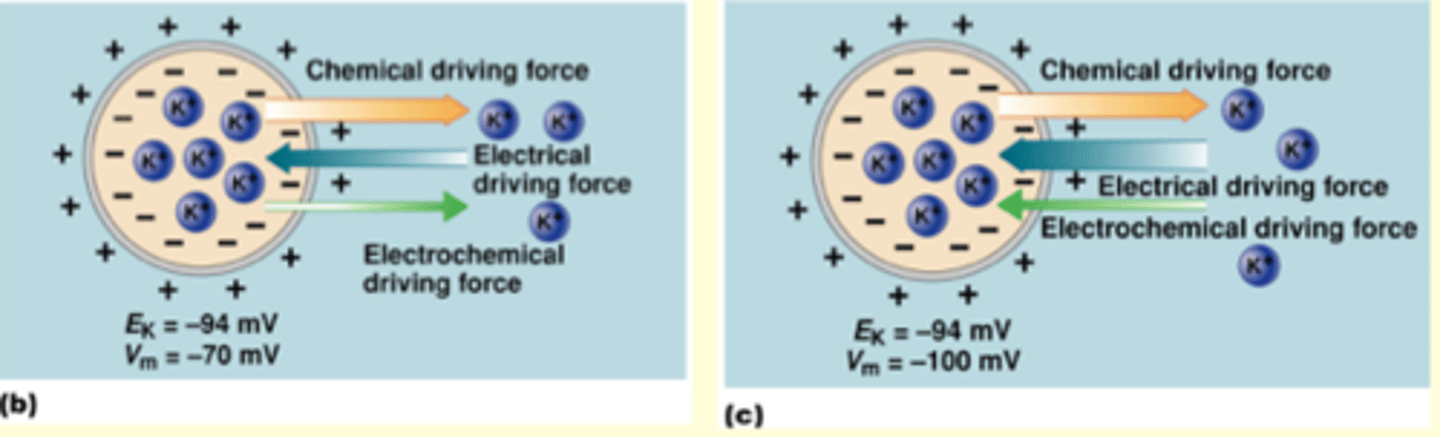
Ei = what the cell would like to be
Vm = what the cell actually is
Anion Q has an equilirbium potential of -60 mV. Q is located in greater concentration (inside/outside) of cell. When a cell is at rest (-70 mV), the direction of the electrochemical force acting on Q is to move it (into.out of) the cell? If channels for Q suddenly opened, the membrane potential would become (more negative/less negative or not change)?
Chemical driving force:
Electrical driving force: going outside of the cell
movement ions: affects electical concentration
cannot change concentration gradient with small fluctuations of ions (cell is bigger)
E: goes outside cell
C: goes inside cell
(a) Since Q is in greater location and Chemical driving force is going outside then Q is outside
(b) Therefore, at rest, you want to make the cell less negative (move ion outside of cell) - electrical arrow is BIGGER because it wanted to get out of cell at -60 mV, it will bigger at -70 mV and cell is not at equilibrium
(c) gonna become less negative bc it wants to go to the less negative equilibrium potential
When is energy required to move a substance across a membrane?
Anion Q will move spontaneously (without the input of energy) in the direction of its electrochemical driving force.
What if Anion Q misbehaves?
We have determined that the electrochemical driving force is outward, but what if we record the flux of anion Q and find that it moves into the cell???
What must be happening?
- transport required (against electrochemical driving force)
Active and Passive transport
ions: require a channel/protein to get them through lipid bilayer and require energy
charged solute: it would electrochemical gradient (not just concentration)
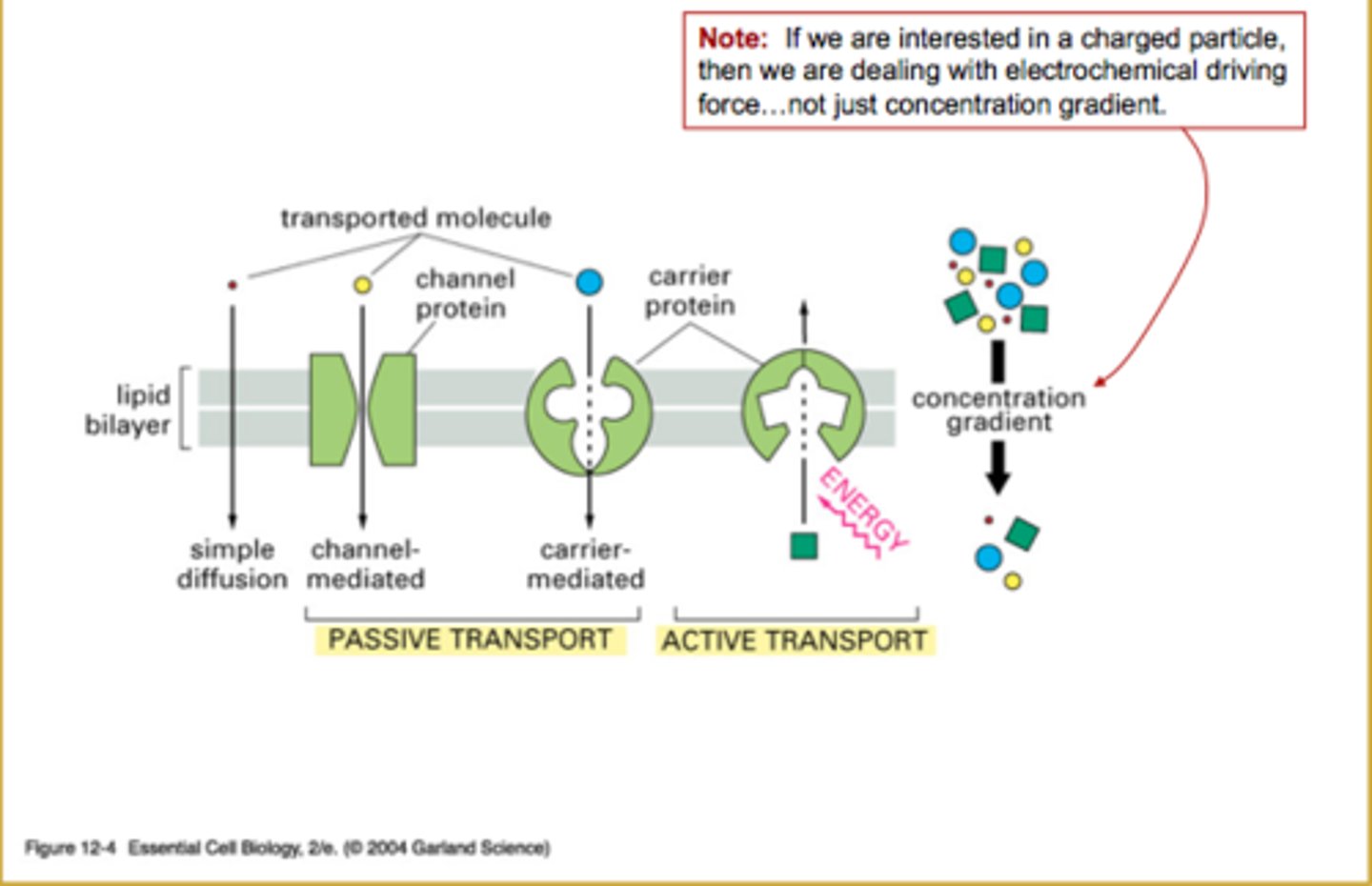
Passive Transport
- found in powerpoint along with ACTIVE
What is the osmolarity of 2 M CaCl2?
6 moles/L
What is the osmotic pressure of 2M CaCl2?
pi = CRT
pi = 6 moles/L • 0.082 • K•atm/K•mol• 310.15 K
pi = 134.4 atm
What is the osmolarity of 0.1M sucrose?
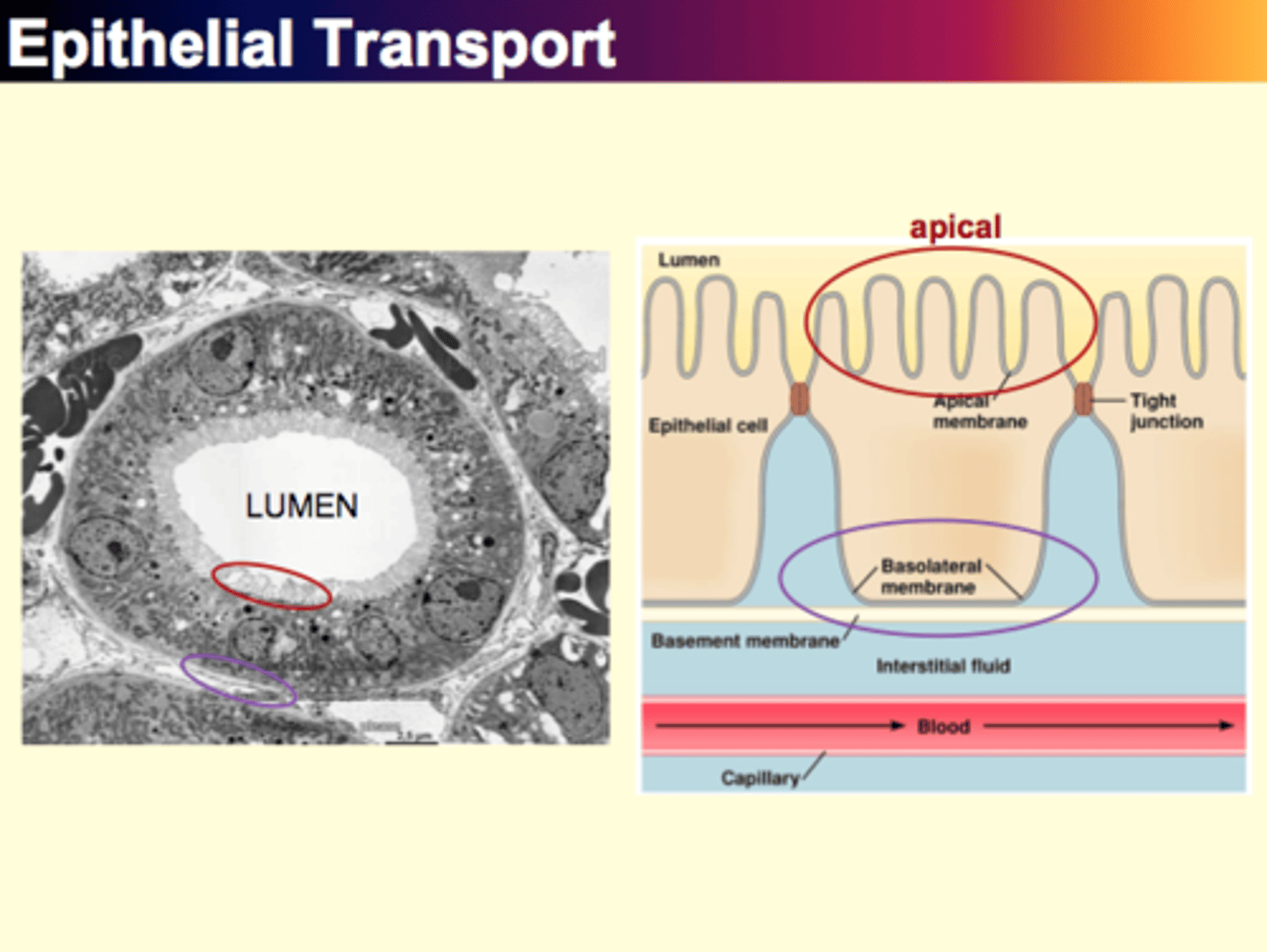
Epithelial Transport
Different, 2 membranes, basal lateral and _
What is are preganglionic cells?
Neurons that travel from the CNS to the ganglia
- synapse with postganglionic neurons in the sympathetic chain
- synapse with postganglionic neurons in a collateral ganglion
- innervates chromaffin cells of the collateral ganglion
What are postganglionic cells?
Neurons that travel from the ganglia to the effector organs
-
What are autonomic ganglia?
Neurons that communicate with one another through synapses located in peripheral structures
What are collateral ganglia?
Preganglionic neurons that synapse with postganglionic neurons
- situated between CNS and the effector organ
What is epithelia?
Tissue (in which epithelial cells are found) which consist of a continuous, sheetlike layer of cells in combination with a thin underlying layer of noncellular material called a basement membrane
Where are epithelial cells found?
Wherever body fluids must be kept separate from the external environment (ie. lining of lungs/skin surface)
Can also be found in the linings of hallow organs
What is the lumen?
The interior cavity of hollow organ or vessel
What is the difference between exocrine gland and endocrine gland?
Exocrine gland secretes a product into a duct leading to the external environment (ie. sweat/salivary gland)
Endocrine glands secrete hormones into the blood stream
What is connective tissue?
Primary function is to provide physical support for other structures, to anchor them into place or to link them together
- many cases, consists of widely scattered cells embedded in a mass of noncellular material called the extracellular matrix
- most diverse type of tissue
- includes blood cells, bone cells, fats cells, etc. (have little to have in common in terms of structure or function)
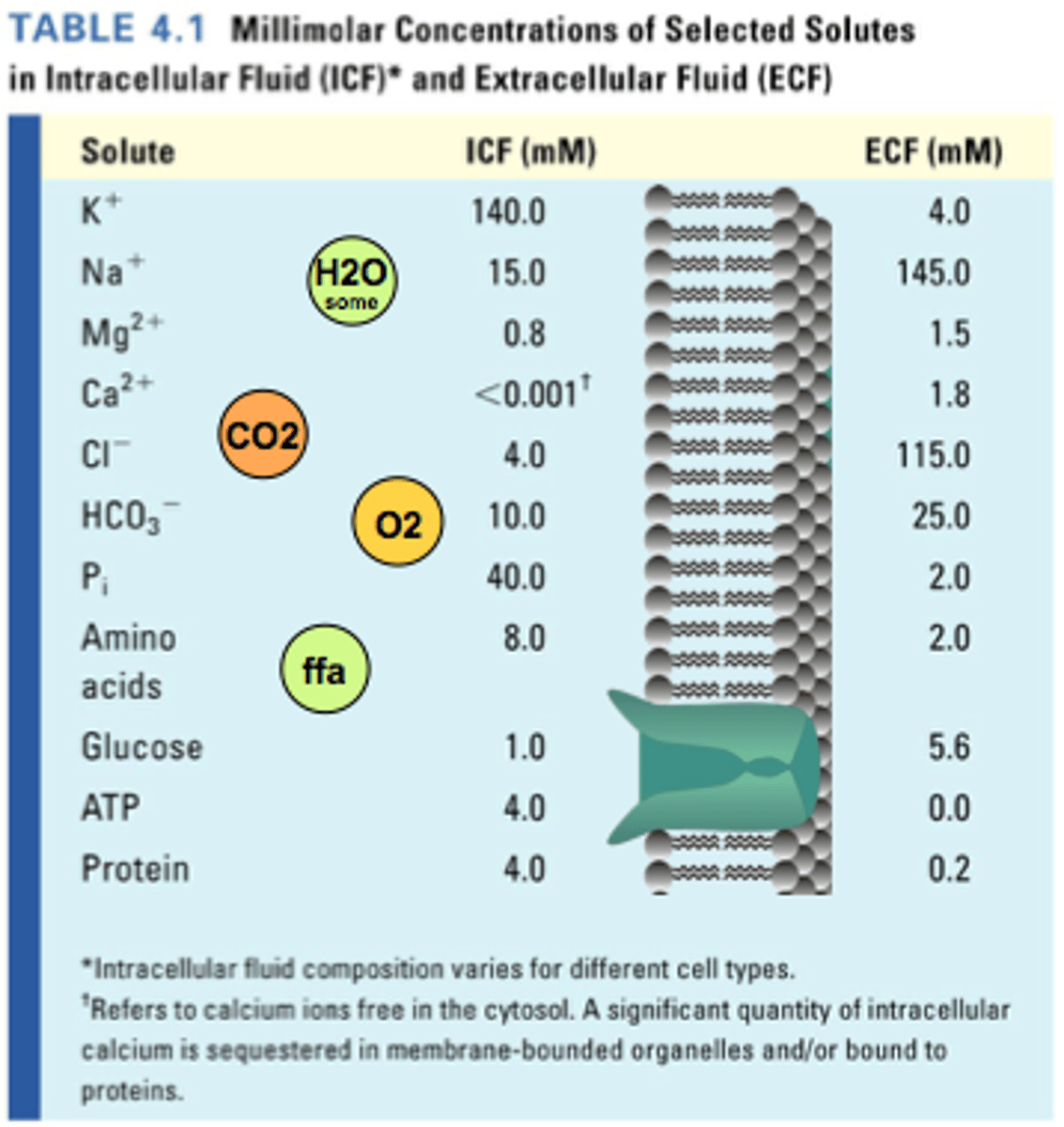
What is meant by the terms, Total Body Water (TBW), Intracellular Fluid (ICF), and Extracellular Fluid (ECF)?
T: total volume of fluid enclosed in the outer epithelial layer; includes both ICF and ECF
I: fluid located inside cells (2/3) - contains many proteins and is relatively rich in potassium
E: fluid located outside cells (1/3) - contains few proteins and (interstitial) is relatively rich in sodium and 20% is found in blood
What is plasma and interstitial fluid?
Plasma is the extracellular portion of fluid that is present in blood (3%)
Interstitial fluid (ISF) is the extracellular portion that is present outside the blood and that bathes most of the cells in the body (11%)
- very similar in composition
- plasma is rich in proteins
What is a negative feedback system?
If a regulated variable increases, the system responds by making it decrease; if it decreases, the system responds by making it increase
What are forms of Passive Transport?
Simple Diffusion: movement of molecule into/out of the cell by its own thermal motion (random) - individual molecules move randomly, populations move down concentration gradient
- Net Flux = size of concentration gradient
- depends on the magnitude of the driving force, membrane surface area, and membrane permeability
Facillitated Diffusion: uses a carrier, which undergoes a conformational change and that is specific for a molecule/class of molecules
- Net Flux depends on frequency of binding
- affects binding: (1) affinity of binding site (2) concentration gradient
- ONLY available from one side
Ion Channel Diffusion: depends on the transport rate of channels and how many open channels there are
- accessible from both sides
- absence of gradient: no net flux
- presence of gradient: net flux down the gradient
What is the Flux?
The rate at which a substance (the number of molecules) is transported across a membrane, in a given length of time
- mole/s
What is Diffusional Equilibrium?
Concentration is the same on both sides, so molecules have same likelihood of crossing to the other side in any given length of time, no net transfer of molecules and the concentrations do not change
What are Aquaporins and how do they work?
They are highly selective pores that permit water but no solutes across the membrane via diffusion
- most water diffuses through this method
- some types are less selective than others (ie. aquaglyceroporins)
- pores are available from both sides
What are the 2 types of Active Transport?
Primary Active Transport: uses ATP/energy directly to transport substances
Secondary Active Transport: powered by a concentration/electrochemical gradient that was previously created by primary active transport
- active can transport molecules in the preferred direction
- depend only one affinity (not concentration)
What is osmosis?
The flow of water down its concentration gradient
What is transcytosis?
Involves endocytosis and exocytosis
- takes in molecule by endocytosis
- endocytotic vesicle does not fuse with a lysosome
- travels to the opposite side to fuse with the plasma membrane and is released via exocytosis
- process in which macromolecules cross epithelial cells
What is Epithelial Water Transport?
- absorb or secrete water by first using active transport of solutes to create osmotic pressure (gradient)
- water flow across epithelium passively by osmosis (secondary solute transport)
1. epithelial actively transport molecules across basolateral membrane into interstitial fluid
2. solute concentration slightly higher outside than inside; osmosis
glucose homeostais answers