Bio Unit 1 The Chemistry of Life
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
element v compound
element
pure substance
can’t be broken down by ordinary means
ex: hydrogen, nitrogen
compound
2 or more different elements combined in a fixed ration
ex: h20, co2
elements of life
carbon, hydrogen, nitrogen, oxygen, phosphorous, sulfur
how to read element chart
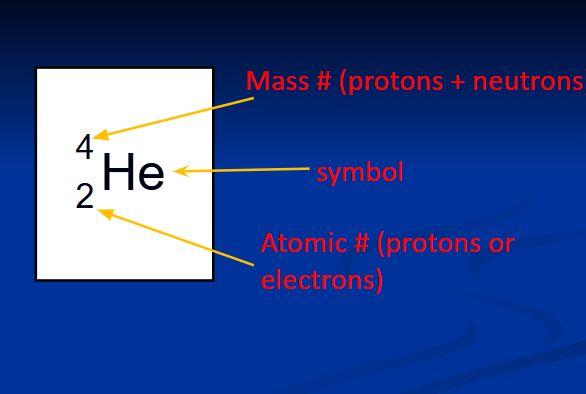
isotopes
number of neutrons varies, but same number of protins
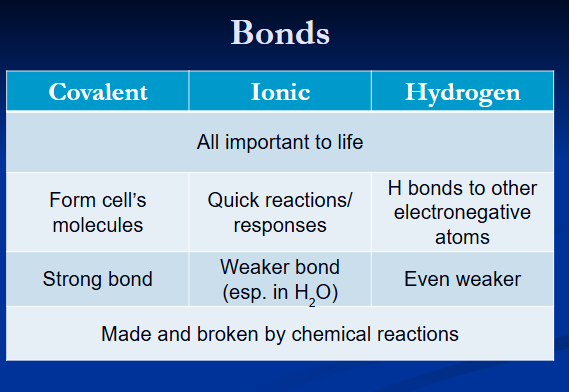
covalent bonds
strong bonds
sharing of electrons
polar and nonpolar
polar covalent bonds
between atoms that differ in electronegativity
ex: h20
nonpolar covalent bonds
electrons shared equally
exL 02, H2
ionic bonds
transfer of electrons between atoms
2 ions bond between givers and takers
ex: Na+Cl-
affected by environment
hydrogen bond
weak bond
h of polar covalent molecule bonds to electronegative atom of other polar covalent molecules
van der waals interactions
weakest bond
slight, fleeting attractions between atoms and molecules close together
ex: gecko toe hairs + wall surface
purposes of different bonds
chemical reactions
reactants → products
some are reversible
chemical equibilibrium
point at which forward and reverse reactions offset one another exactly
no net change in concentrations of reactants/products
radioactive isotopes
An unstable form of a chemical element that releases radiation as it breaks down and becomes more stable. Radioisotopes may occur in nature or be made in a laboratory. In medicine, they are used in imaging tests and in treatment.
valence electrons
the electrons in the outermost or valence shell, are important
provide insight into an element's chemical properties and are the ones gained, lost, or shared during a chemical reaction. In general, atoms are most stable and least reactive when their outermost electron shell is full.
single v double bond
A single bond involves one shared pair of electrons between two atoms, while a double bond involves two shared pairs of electrons between the same two atoms. Single bonds are longer and weaker than double bonds.
hydroxyl group
-OH
alcohol
specific name usually ends in -ol
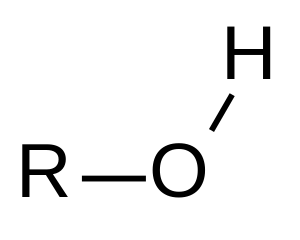
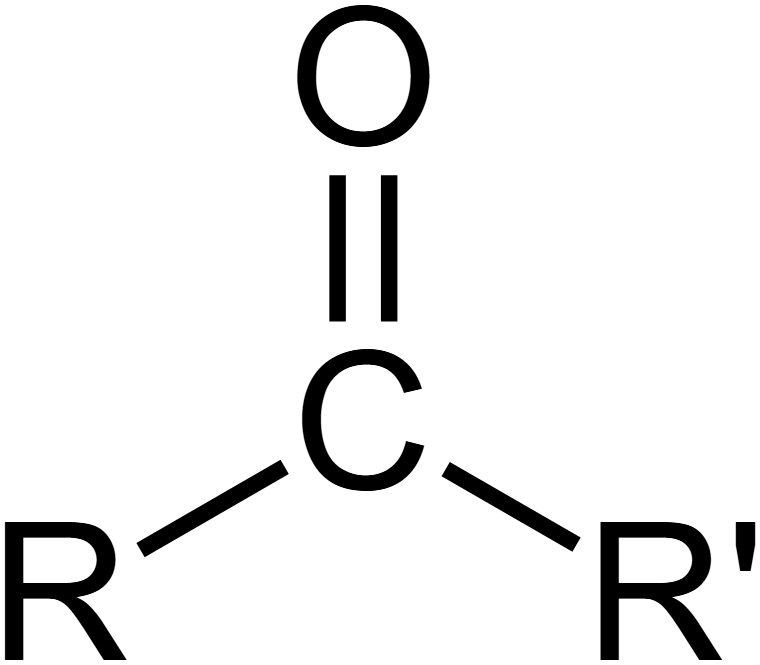
carbonyl group
sugars
compound name: ketone or aldehyde (if carbonyl group is at end of a carbon skeleton
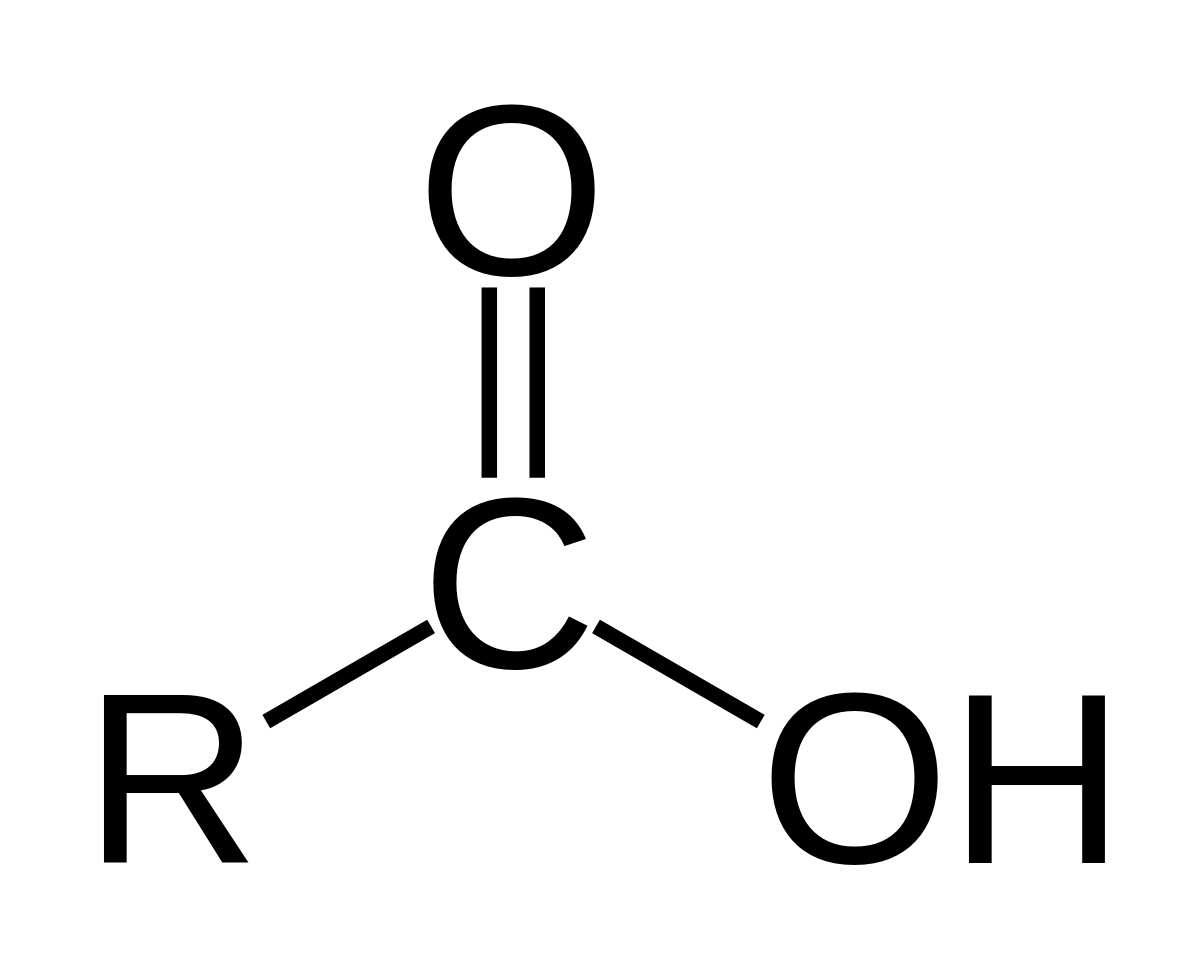
carboxyl group
-COOH
acts as an acid (can donate H+)
compound name: carboxylic acid or organic acid
amino group
-NH2
compound name: amine
acts as a base
sulfhydryl group
-SH
two sulfhydryl groups can react to form a “cross link” that helps stabilize protein structure
compound name: thiol
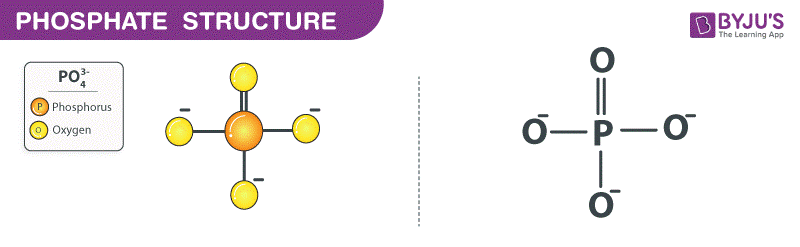
phosphate group
-OPO3
contributes negative charge
compound name: organic phosphate
methyl group
-CH3
affects the expression of genes when on DNA or on proteins bound to DNA
compound name: methylated compound
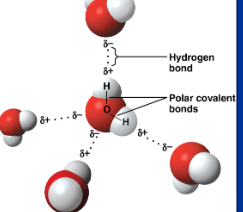
water is a polar/nonpolar molecule
polar
why is water polar?
because of the unequal sharing of electrons between oxygen and hydrogen since oxygen has a higher electronegativity than hydrogen and h20 has a bent/assymmetrical shape
in h20, region around oxygen has a partial ? charge, region near the hydrogen has a partial ? charge
negative, positive
bonds in H20
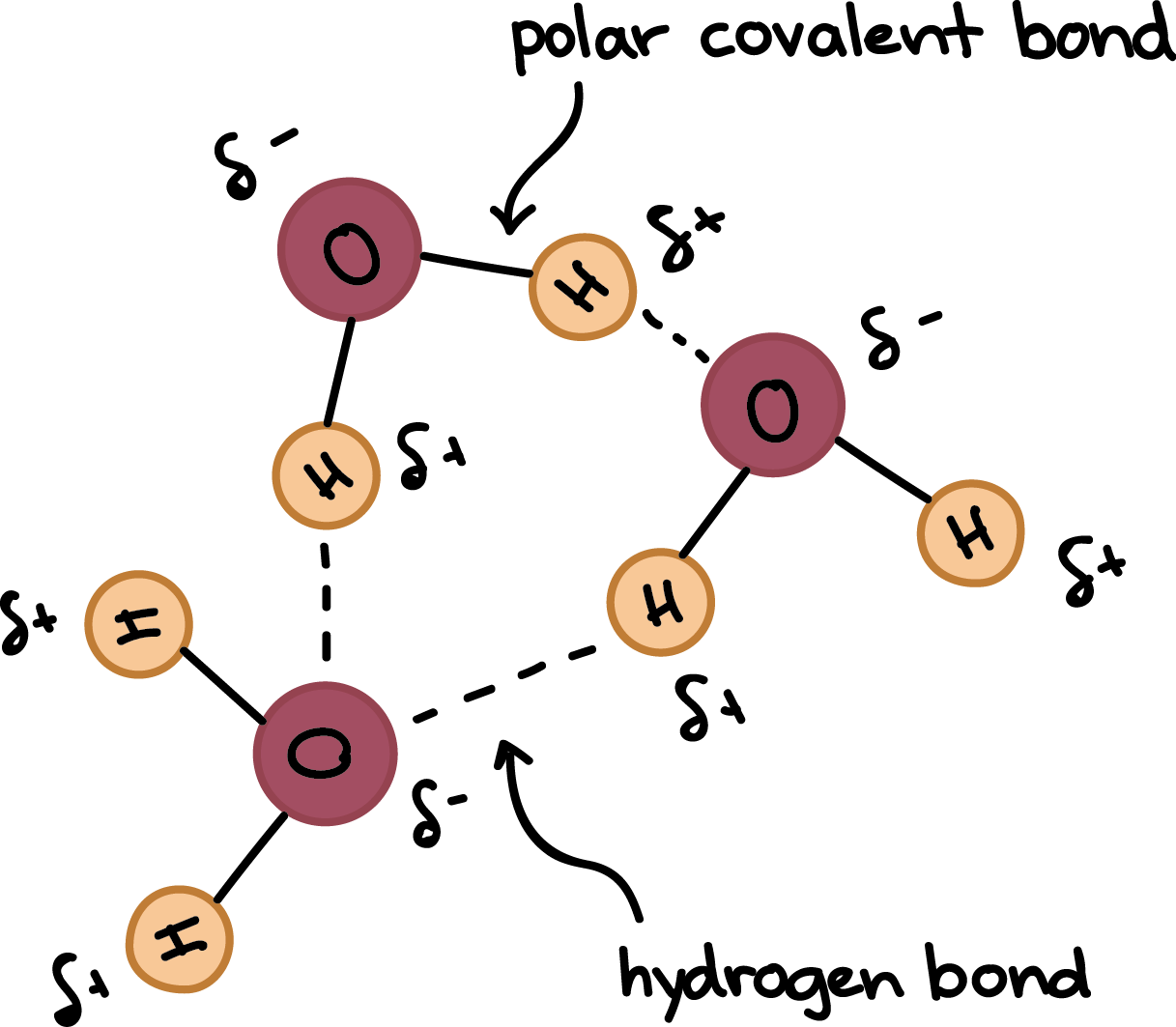
cohesion
hydrogen bonding between like molecules (polar)
ex: surface tension - measure of how difficult it is to break or stretch surface of liquid
adhesion
hydrogen bonding between unlike molecules
ex: capillary tubes, water & plants; water is attracted to the glass walls of the tube so water is able to “climb upwards through the capillary tubes. this counters the downward pull of gravity
transpiration
movement of H20 up plants and evaporation of water from leaves and evaporation of water from leaves
cohesion and adhesion occurs
thermal energy (heat)
the total amount of kinetic energy in a system
temperature
measure intensity of heat due to the average kinetic energy of molecules
high specific heat of water
it takes a significant amount of energy to raise its temperature compared to other substances
most of the heat is used to disrupt hydrogen bonds
changes temp less when it absorbs or loses heat
larger bodies of water absorb and store more heat
a person swimmming in a pool has a higher temperature than the pool but the water has a higher amount of heat
helps regulate temperatures on Earth and in living organisms
water has a high heat of vaporization
requires a large amount of energy to transition from a liquid to a gaseous state
this is due to the strong hydrogen bonds in water
evaporative cooling
molecules with greatest kinetic energy leave as gas (molecules are moving fast enough to overcome attractions of like-like molecules)
this is a reduction in temperature resulting from evaporation of liquid
ex: human sweat
insulation by ice: ice is less dense
when water freezes, the molecules expand due to hydrogen bonds, sicne the molecules move slower, the kinetic energy decreases
the molecules move further apart so it becomes less dense
hydrophilic
affinity for H20
polar, ions
cellulose, sugar, salt, blood
hydrophobic
repels H20
non-polar
oils, lipids, cell membrane tails
water dissociation
h20 breaks into H+ (acts as acid) and OH- (acts as base)
reverisible equilibrium reaction
acids ? H+ concentration
bases ? H+ concentration
increase, reduce
pH formuka
pH = -log(H+)
(H+)(OH-) = 10^-14
if H+ = 10^-2 -log(10^-2) = -(-2) = 2'
if OH- = 10^-10 H+ = 10^-4 -log(10^-4) = -(-4) = 4
buffers
minimize changes in concentrations of H+ and OH_ in a solution (makes weaker acids and bases)
carbonc acid-bicarbonate buffer system - a critical mechanisms for maintaining a stable pH in the blood and other body fluids (blood has to be at pH ~7.4)
4 emergent properties of water
cohesive/adhesive behavior
ability to moderate temperature
expansion upon freezing
versatility as a solvent
heat must be ? in order to break hydrogen bonds
heat must be ? when hydrogen bonds form
absorbed
released
water is a universal solvent
due to polarity
can dissolve ionic compounds because the partially negative charge of the oxygen attracts cation and the hydrogen is attracted to anions
carbon has ? valence electrons so it can form up to ? covalent bonds
4
4 classes of macromolecules
carbohydrates
proteins
lipids
nucleic acids
isomers
molecules have same molecular formula, but differ in atom arrangement
structural: Differ in the covalent arrangement of atoms and often in the location of double bonds.
geometric: Same sequence of covalently bonded atoms but differ in spatial arrangement due to the inflexibility of double bonds
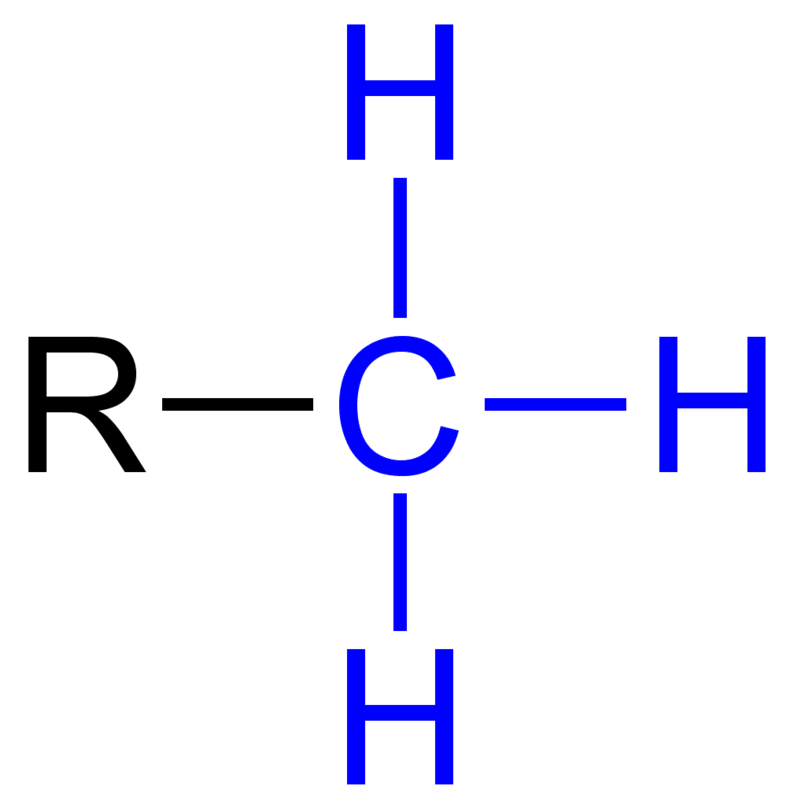
hydrocarbons
organic molecules consisting of any carbon and hydrogen. they are major components of petroleum. they can undergo reactions that release a relatively large amount of energy
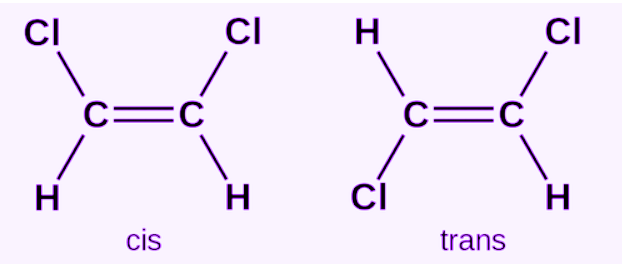
cis-isomers
same atoms attached to double bonded carbons on the same side of the double bond
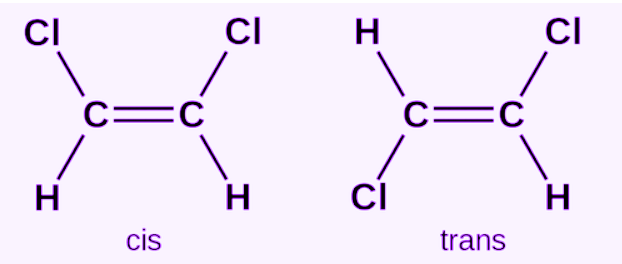
trans isomer
Unlike Cis, this has atoms attached on opposite side of double bond
enatiomers
isomers that are mirror images of each other and differ in shape due to the prescence of an asymmetric carbon
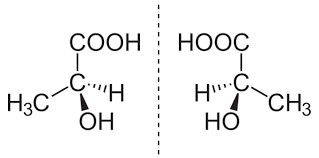
asymmetric carbon
covalently bonded to 4 different kinds of atoms or groups of atoms, whose arrangements can result in mirror images
polymer
a long molecule consisting of many similar or identical building blocks, linked by covalent bonds
monomers
building blocks of polymers
dehydration synthesis
a reaction in which 2 molecules are covalently bonded to each other with the loss of a water molecule
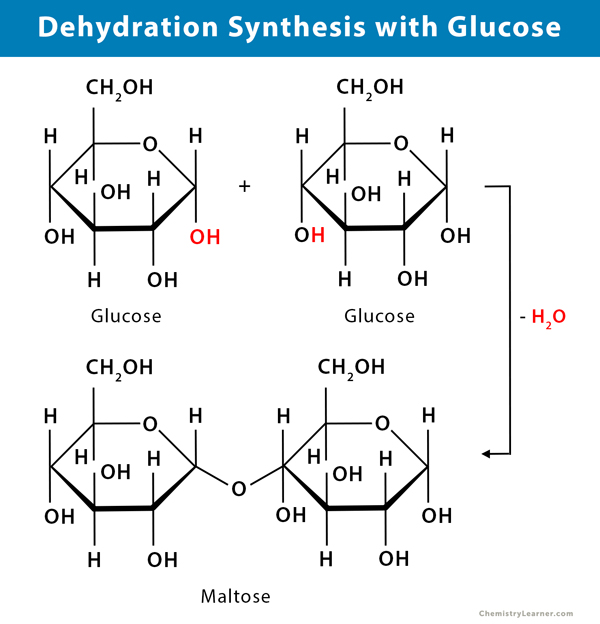
polymerization
the repetition of monomers added to the chain, making a polymer
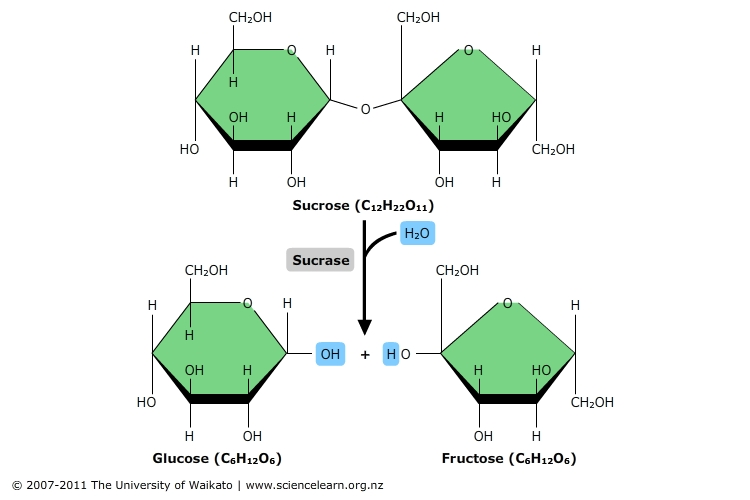
hydrolysis
polymers are disassembled to monomers by the addition of a water molecule
carbohydrates
include sugars and polymers of sugars
monosaccarides
carbohydrate monomers
ex: glucose, fructose, galactose
disaccharides
double sugars with 2 monosaccharides joined by a covalent bond
ex: maltose, lactose, sucrose
most stable form of sugars
rings
cellular respiration
cells extract energy from glucose molecules by breaking them down in a series of reactions
glycosidic linkage
the covalent bond formed by a dehydration reaction
because disaccharides are joined together by dehydration synthesis, they have the chemical formula of ? bcause one hydrogen is removed
C12H22O11
polysaccharides
many monosaccharides joined by glycosidic linkage
some are used for storage, some are used as uilding material for structures that protect the cell
ex: starch (storage), cellulose (protection for planst), glycogen (storage for animals), chitin (insect protection)
examples of lipids
fats, oils, phospholipids, waxes, steroids, fat soluble vitamins
fat
constructed from the monomers glycerol and fatty acids
fatty acid structure
long carbon skelton, usually 16 or 18 carbon atoms
end of skeleton has functional group carboxyl
rest of skeleton consists of a hydrocarbon chain
fatty acids are hydrophobic because:
nonpolar C-C bonds in the hydrocarbon chains
ester linkage
bond formed by a dehydration reaction between an -OH group and a carboxylic acid
joins fatty acid molecules to glycerol
triacylglycerol
3 fatty acids connected to one glycerol
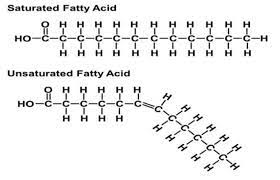
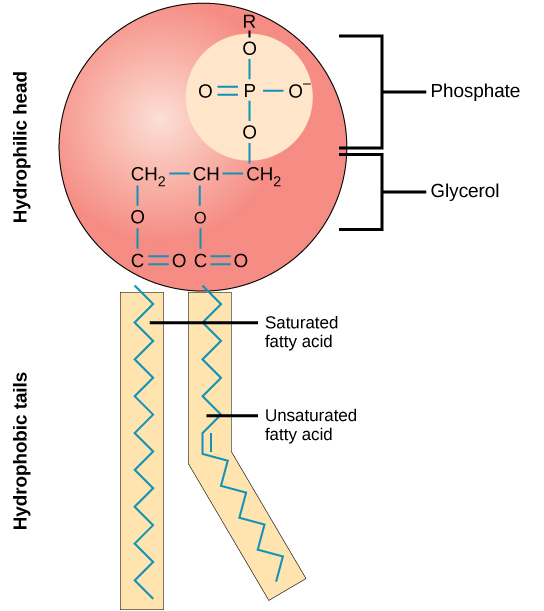
saturated fatty acids
no double bonds between carbon atoms
typically solid; ex: butter and waxes
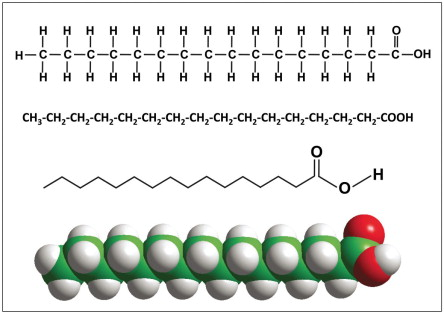
unsaturated fatty acids
have one or more double bonds with one fewer hydrogen on each double bond
bend in hydrocarbon chain caused by double bond
typically liquid; ex: oils
main function of fats
energy storage
phospholipids
making up a huge component of the cell membrane
made up of 2 fatty acids, a glycerol and a phosphate group
hydrophilic head and hydrophobic tail
protein
a biologically functional molecule made up of polypeptides into different structures
ex: enzymatic, defensive, storage, transport, hormonal, receptor, motor, structural
proteins are all constructed from the same set of ? amino acids
20
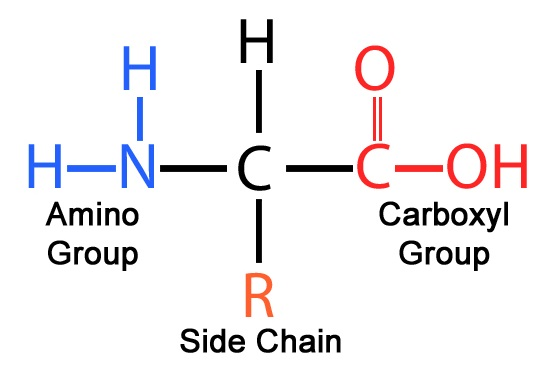
amino acids
building blocks/monomers of proteins
made up of amino group and carboxyl group
polypeptide
polymers of protein
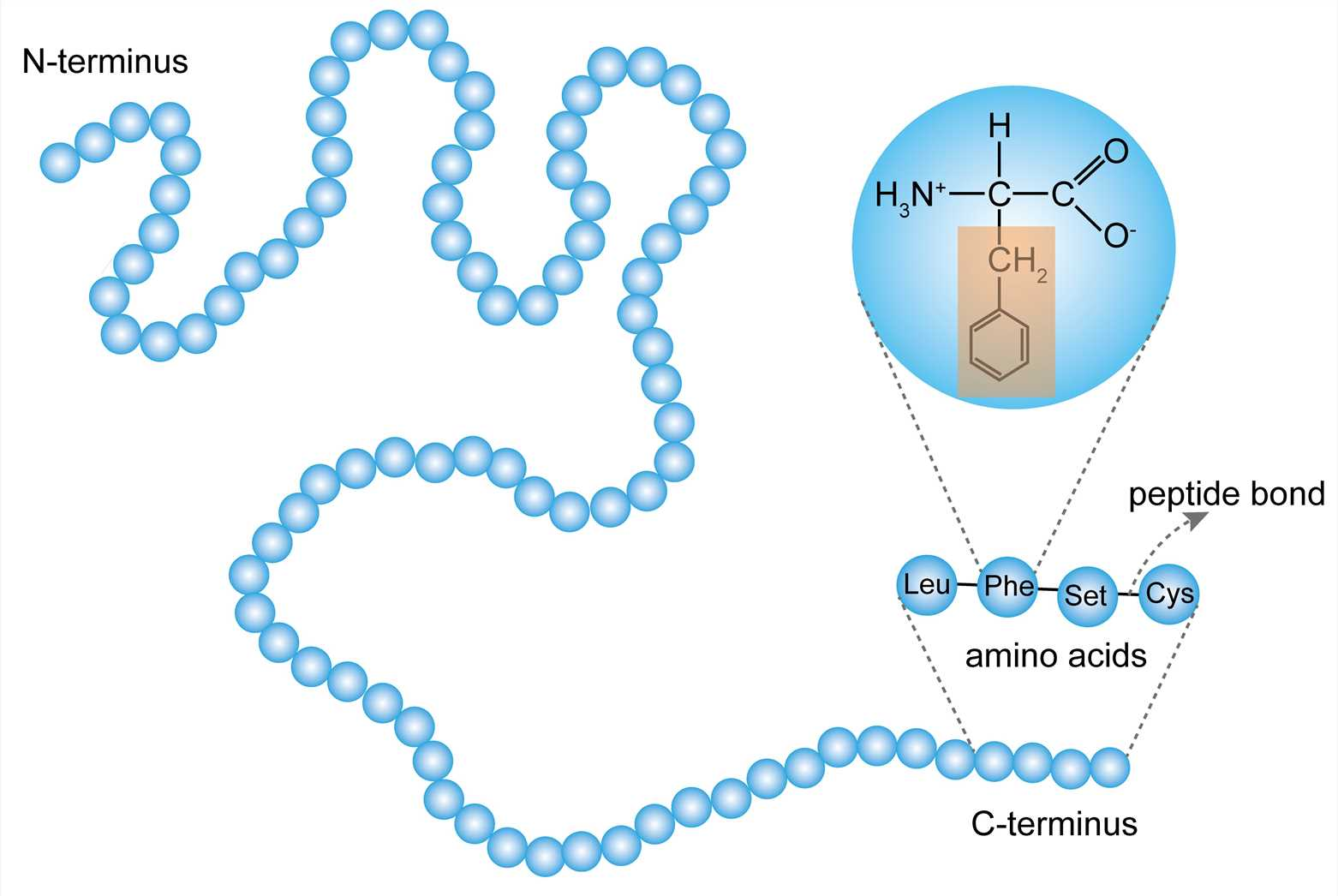
peptide bond
bond that connects amino acids
covalent bond formed between the carboxyl group of one amino acid and the amino acid group of another
importance of side chains
physical/chemical properties of the side chain deterime the unique characteritics of an amino acid
nonpolar side chains are hydrophobic amino acids
polar side chains are hydrophilic
acidic amino acids have side chains that are negative charged (due to carboxyl) (hydrophilic)
basic amino acids have positive charge (hydrophilic)
polypeptide backone
the repeating sequence of amino acids
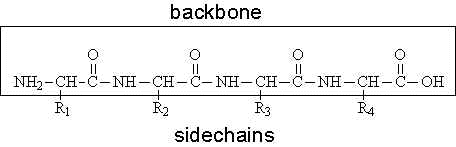
primary protein structure
a linear chain of amino acids. it has an amino and carboxyl end and the precise structure is determined by inherted genetic infromation
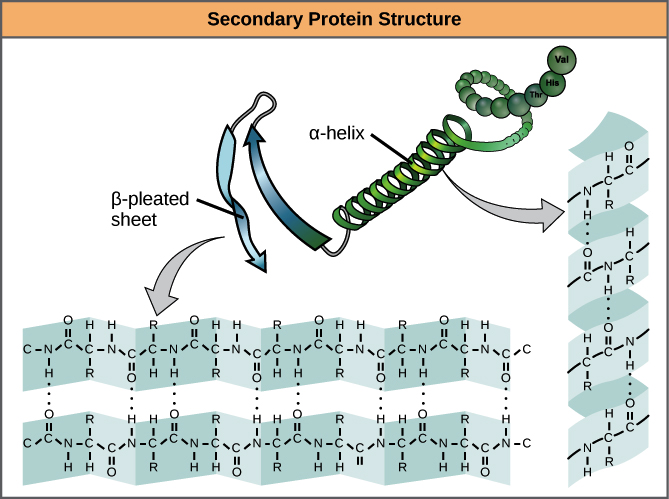
secondary protein structure
region stabilized by hydrogen bonds between atoms of the polypeptide backbone.
segments of polypeptide chains repeatedly coiled (to make a helix) or folded (to make a pleated sheet)
tertiary protein structure
3d shape; stabilized by interactions between side chains (hydrogen, ionic, van der waals, disulfide bridges)
hydrophobic side chains usually end up in the clusters at the core
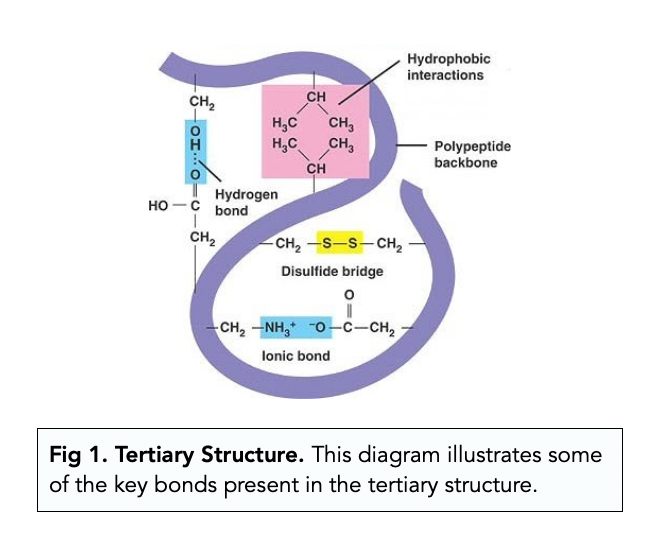
disulfide bridge
strong covalent bond between suflfur atoms of two cysteine amino acids
helps stabilize tertiary protein’s 3d structure
quaternary structure
association of 2 or more polypeptides
ex: collagen, hemoglobin
factors that can lead to protein denaturation
pH, temperature
denaturation
protein is now biologically inactive, caused by chemicals that disrupt bonds
monomer of nucleic acids
nucleotides
gentic information flows from:
DNA → RNA → protein
DNA
deoxyribonucleic acid
carries genetic instruction, directions for its own replication, controls protein synthesis
messenger RNA (mRNA)
carries genetic information from the DNA in the nucleus to the ribosomes in the cytoplasm where the proteins are synthesized
where does protein synthesis occur?
ribosomes
polymers of nucleic acids
polynucleotides
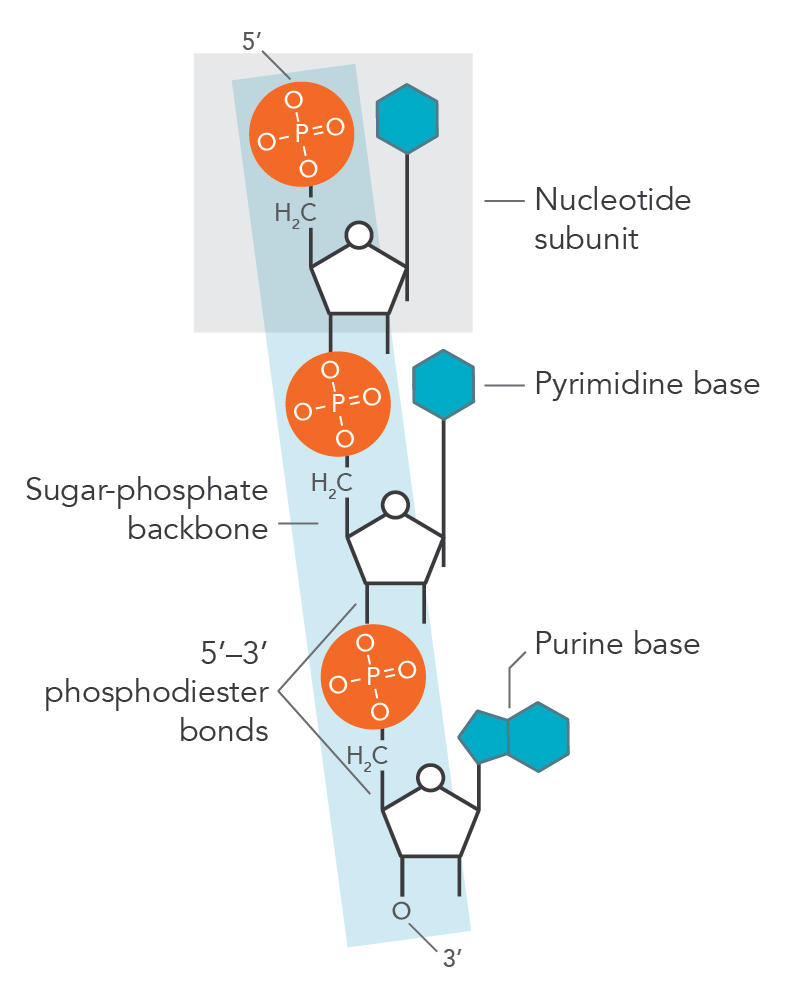
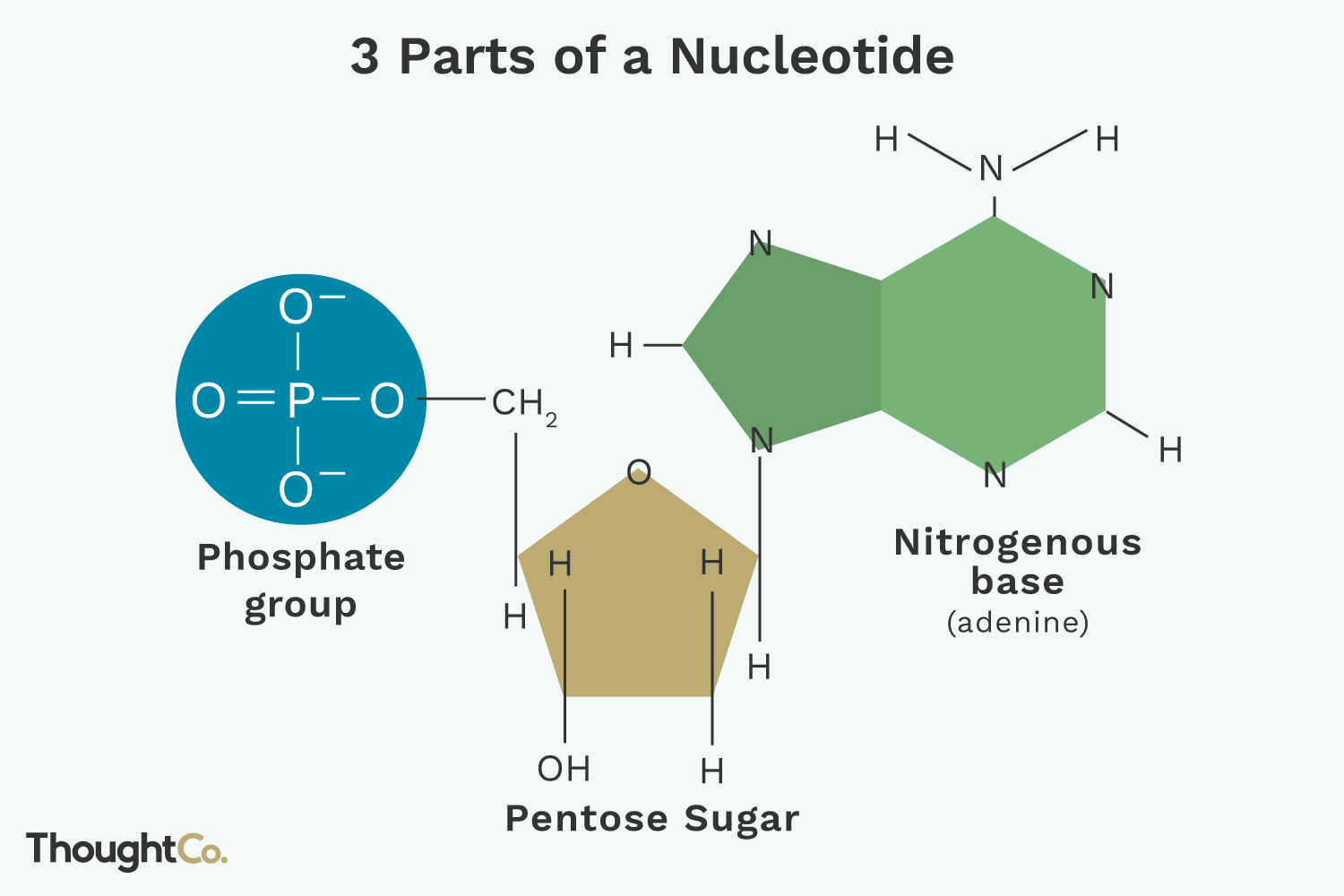
structure of nucleotide
nitrogenous base, 5 carbon sugar (deoxyribose), and phosphate group
nucleoside
portion of a nucleotide without any phosphate group (base+sugar)
2 families of nitrogen bases
purines (as good as gold) - double ringed
pyrimidines (cut the umbrella)
bond that joins nucleotides
phosphodiester linkage (creates sugar phosphate backbone)