Required practical 5: distillation of a product from a reaction- preparation of an organic liquid
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
Describe the experiment of distillation
add acidified potassium dichromate and sulfuric acid to a flask
Pour mixture into pear shaped flask with still head containing the thermometer
Attached to a condenser with cooled collection vessel
Add anti bumping granules
Heat flask gently
Collect same of boiling point of desired product
Why do we use a cooled collection vessel?
reduces evaporation of the product
Draw the diagram of distillation and label
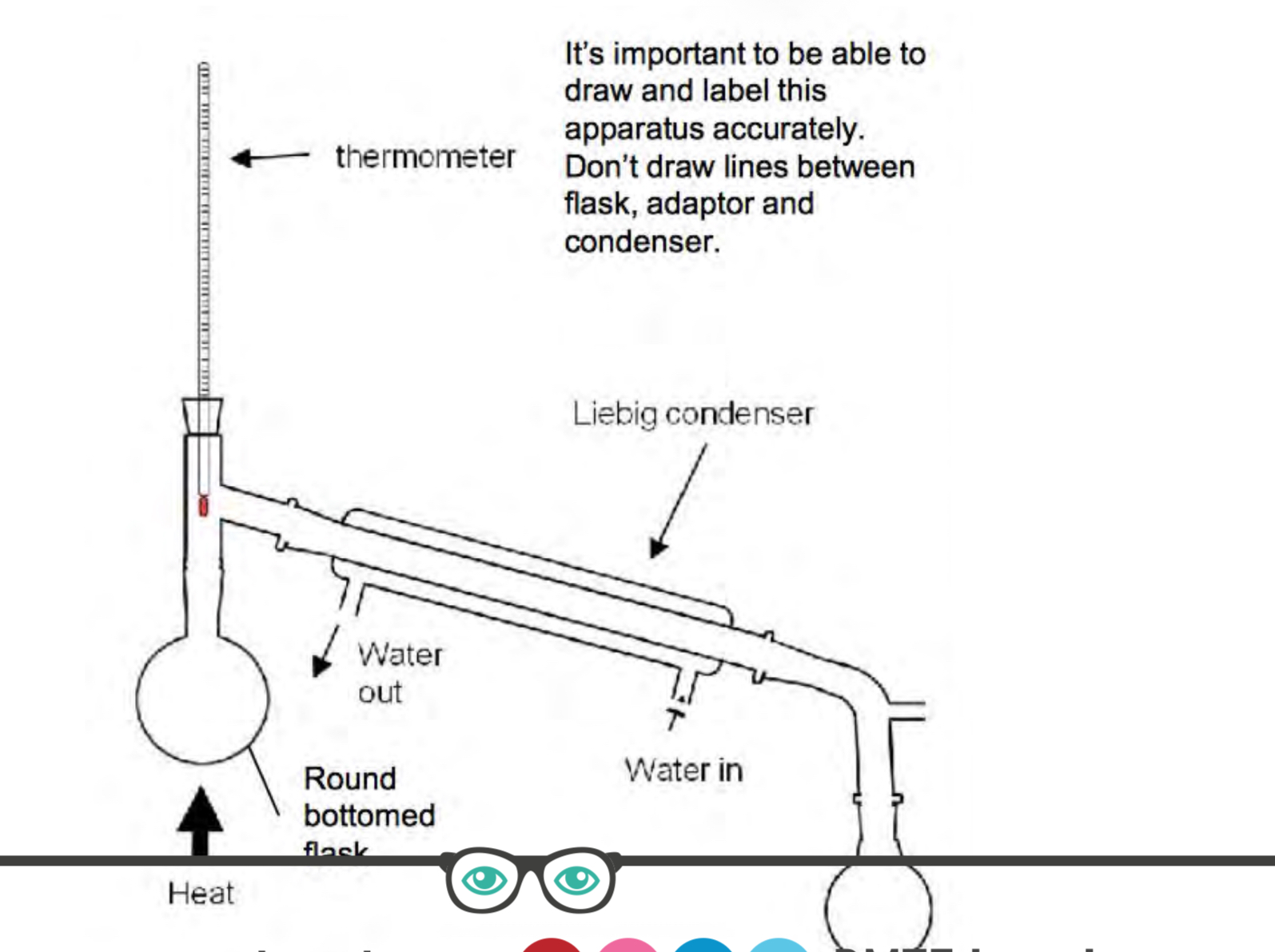
Describe oxidation of primary alcohol to aldehyde experiment
dissolve acidified potassium dichromate in sulfuric acid
Add anti bumping granules and shake to mix contents
Add an alcohol, shake the flask to mix the contents
Keep test tube cool to avoid loss of volatile ethanol
Gently heat
Why does the water go in at the bottom of the distilling apparatus?
goes against gravity as this allowed more efficient cooling and prevent back flow of water
Why are electric heaters used?
alcohols are highly flammable
Why should you never seal the end of the condenser?
build up of gas pressure could cause the apparatus to explode