Chem 261 Reactions
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
SN2 reaction
-bimolecular nucleophilic substitution reactions
- only 1 step (concerted reaction)
-nucleophile attacks the compound at the same time as the leaving group leaves
-Nucleophile actively displaces the leaving group in a backside attack
nucleophile must be strong
substrate is 1 degree alkyl halide
-concentrations of substrate & nucleophile have role in determining the rate --> rate = k[Nu][R-L]
stereochemistry inverted
-polar aprotic solvent
Sn1
-secondary or tertiary alkyl halides
-no stereochemistry defined
popar protic solvent
carbocation rearrangements possible
-carbocation is formed by leaving group leaving, then if water is present, it bonds to carbocation and second water molecule can turn it into a OH
-poor nucleophile
-weak base.
E2 reaction
-forms ALKENE
-strong base reacts with hydrogen and kicks off leaving group in a concerted reaction
-anti periplanar
-stereochemistry flips
-tertiary alkyl halide
-polar aprotic solvent
-high temperature
Zaitsev's rule-most substituted alkene is usually preferred unless the base is very bulky ***
E1 reaction
-forms ALKENE
-weak base
-polar protic solvent
-high temperature
-similar to Sn1
-less stereoselective
-a multistep elimination where the leaving group is lost in a slow ionization then a hydrogen is lost in a second step.
Preparing Internal Alkynes
vicinal halides (2 halogens on carbons beside each other on an alkane) + NaNH2 where NH2 depropenates the hydrogen and the halogen is kicked off. this happens twice and forms a internal alkyne **** remember to put the R groups on the side
terminal alkyne
need 3 equivalents of base. after 2 equivalents, you have your terminal alkyne and you can use the NH2 to remove the hydrogen and make this a nucleophile *** Really important TBH
Oxidative Cleavage
1. O3;
2. S(CH3)2 [i.e. DMS]
in this reaction, you are forming 2 groups that contain carbonyls (C=O) by essentially splitting molecule in half
![<p>1. O3;</p><p>2. S(CH3)2 [i.e. DMS]</p><p>in this reaction, you are forming 2 groups that contain carbonyls (C=O) by essentially splitting molecule in half</p>](https://knowt-user-attachments.s3.amazonaws.com/9c26e5e3-d1ff-4908-9ef4-616b02b4f76f.jpg)
Oxidative cleavage using hot potassium permanganate
KMnO4, KOH, H2O, heat
- contains max oxidation of each carbon
remember:
monosubstituted= carboxylate salts
disubsituted: ketones
unsubstituted: carbon dioxide
O3 (ozone)
O=O(+)-O(-)
Dihydroxylation
alkene
+ 1. OsO4;
2. NaHSO3
Makes a Cis-2-diol
Reaction of an alkene with osmium tetroxide followed by treatment with bisulfite in water, results in the addition of a hydroxyl group to either carbon of the original bond
(can also use potassium permanganate under basic conditions at low temperatures)
-syn addition of 2 hydroxyls
Dihydroxylation
alkene
+KMnO4, KOH, H2O, 0oC
Makes a Cis-2-diol
Reaction of an alkene with potassium permaganate under basic conditions at low temperatures
(can also use OsO4 and water)
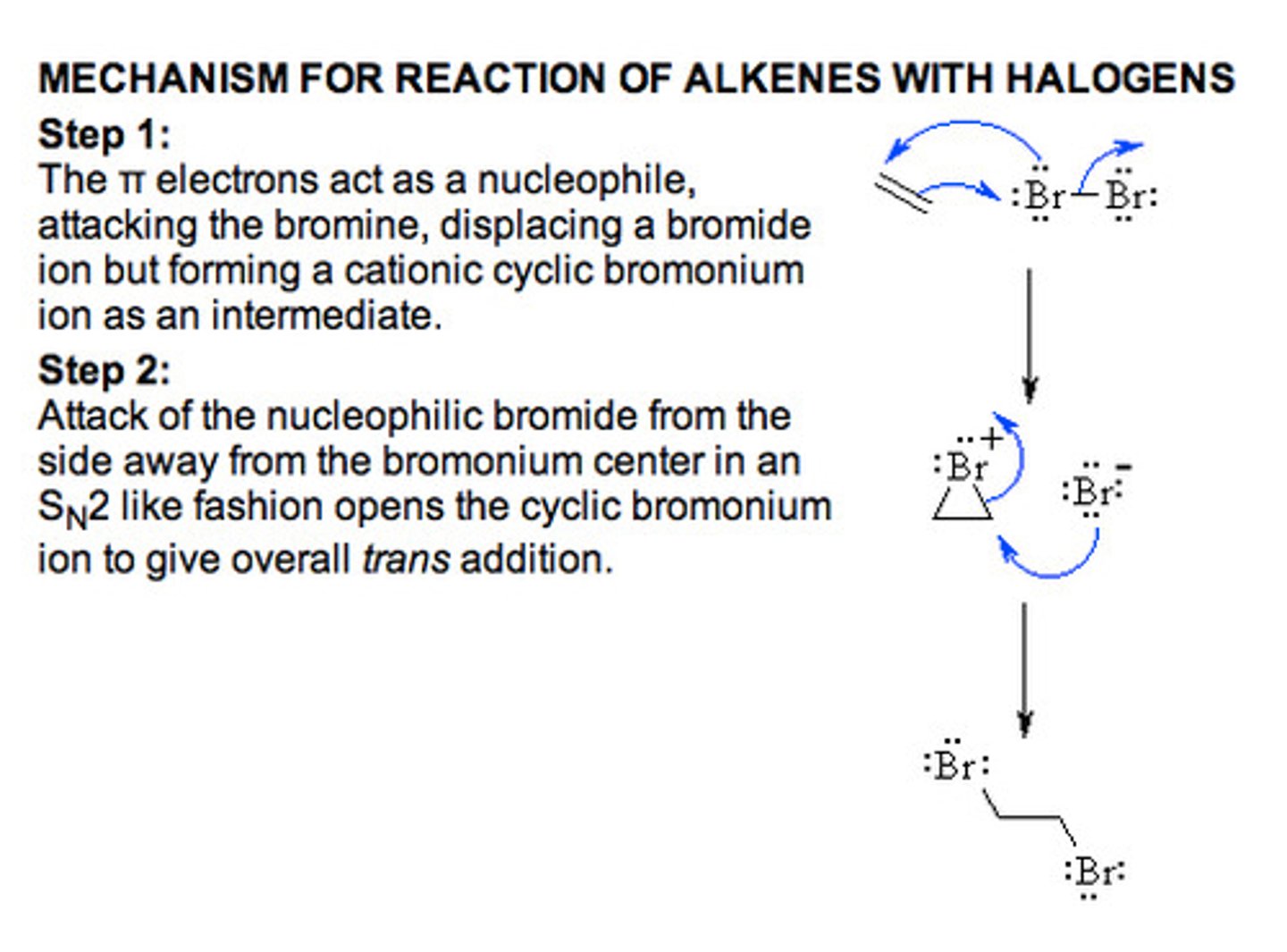
Adding X2 to alkenes
1.Br2 (or Cl2)
This reaction involves the formation of a 3 membered ring that contains the halogen. First X2 reacts with the nucleophilic double bond and bonds to it and forms a ring. The second halogen is kicked off the X2. The ion can then be used to open up the ring ---> SN2 chemistry
-racemic mixture of trans product is formed
end product: trans (Trans-1,2-dibromide)
Adding X2 to alkenes- high concentration of water
1. Br2 (or Cl2), H2O
1 eq of bromine with an excess of water, halonium intermediate will be opened by the water instead of the halogen ion, and a molecule that is trans- with a halogen and a OH is formed. when double bond is unsymmetrical, OH ends up on more substituted carbon
Addition of X2 to alkyne
1 equivalent -anti addition to form trans haloalkane
2 equivalents- forms tetrahaloalkane
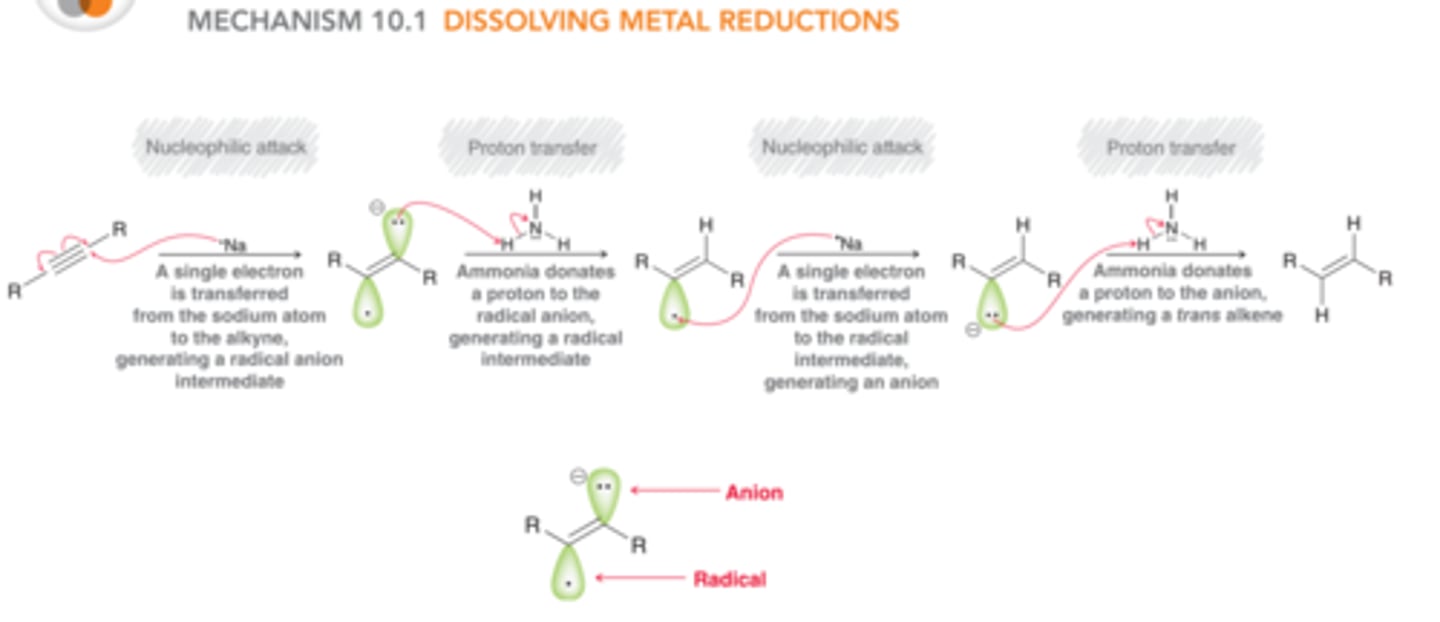
Dissolving Metal Reduction
1. Li or Na, NH3
2. NH4Cl
An awesome reaction that involves the formation of a radical
-An alkyne substrate is subjected to lithium or sodium metal dissolved in ammonia
-alkyne to trans alkene TRANS PRODUCT FORMED
-anti addition
Lindlar's catalyst
A heterogeneous catalyst for the hydrogenation of alkynes to cis alkenes.
syn addition
addition of constituents to an alkene on the same side of the pi bond
Hydrogenation
1. H2
2. Pd/C
reducing double bonds to single bonds
forms cis products
Hydrogenation with Lindlar's Catalyst
1.H2,
2. Pd/C, CaCO3, quinoline
when you don't want to reduce all the way to single bonds you can form a cis alkene--> syn addition
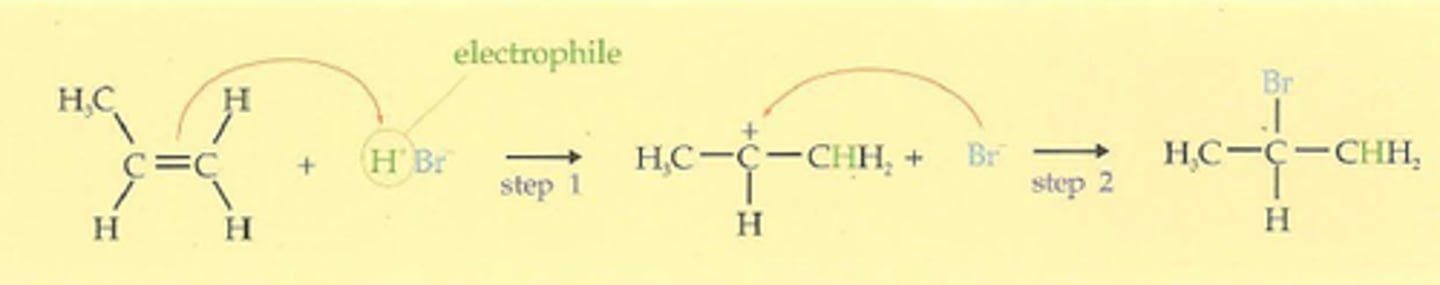
Markonikov's rule
In an addition reaction of a protic acid HX (hydrogen chloride, hydrogen bromide, or hydrogen iodide) to an alkene or alkyne, the hydrogen atom of HX becomes bonded to the carbon atom that had the greatest number of hydrogen atoms already (least substiuted carbon)
Adding H-X to alkene
1. HI or HBR or HCl
addition is markovnikov
reactivity of H-X
increases with larger atoms
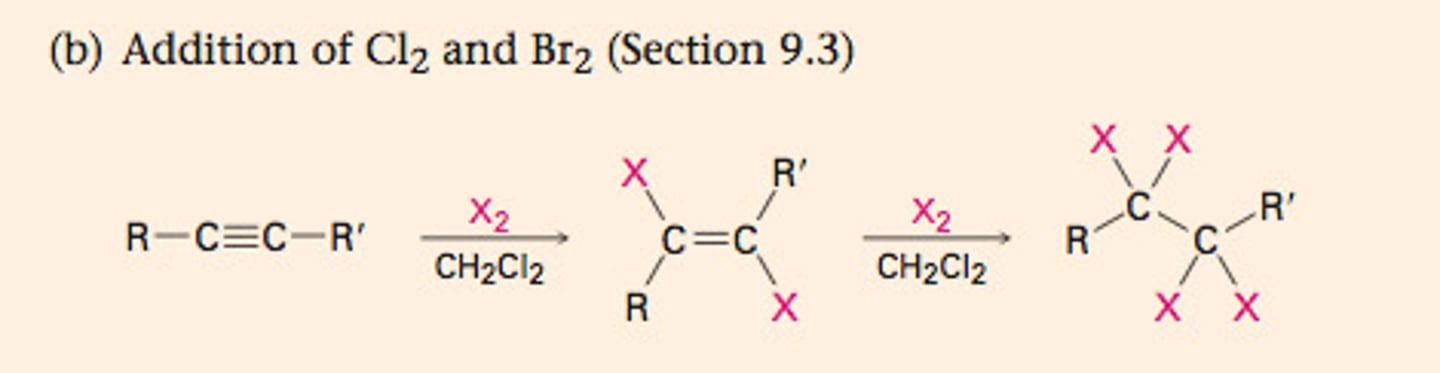
Addition of H-X to alkyne
-1 equivalent (added anti) forms haloalkane and 2 equivalents forms gem-dihalide this is a carbon with two halides bonded to it
Adding H-X to alkene- Acid Catalyzed
1. H2SO4
2. H2O
if you have a alkene and strong acid and a water molecule, the alkene is the nu and will attack hydronium to make carbocation, the carbocation is attacked by the water and then the leaving group leaves the molecule using water.
-markovnikov
-arrangements allowed
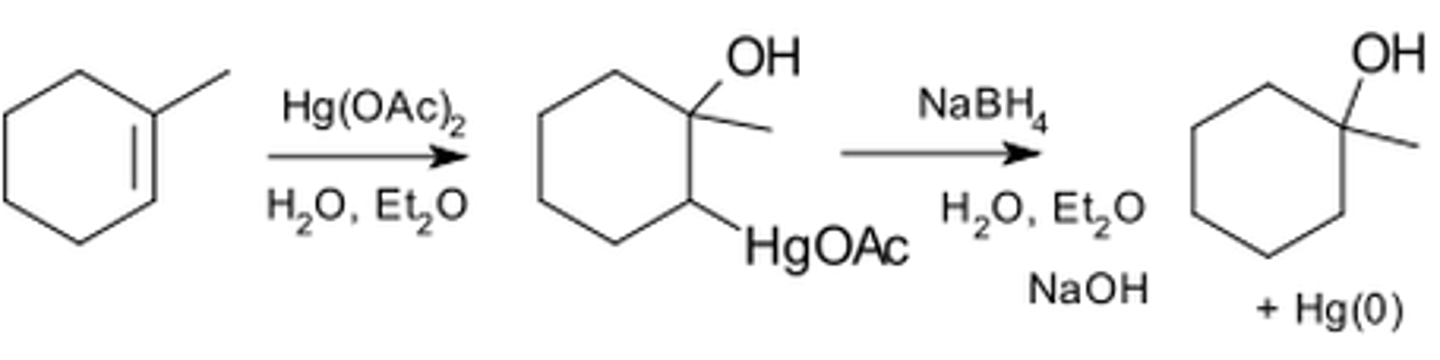
Oxymercuration Demercuration
1. Hg(OAc)2, H2O
2. NaBH4, NaOH
WHAT HAPPENS?
-this process limits rearrangements to the carbocation
-still markovnikov reaction
Hydroboration
1. BH3;
2. H2O2, NaOH
-anti markovnikov- putting the hydrogen on the least substituted carbon (and the hydrogen on the most substituted)
-a reaction that allows the addition of water across a double bond in anti markovnikovs
- reaction mechanism make sure you know: boron bonds to the least substituted carbon
alkoxide ion
full negative charge on oxygen resulting from deproponation of an alcohol
nucleophlillic and basic
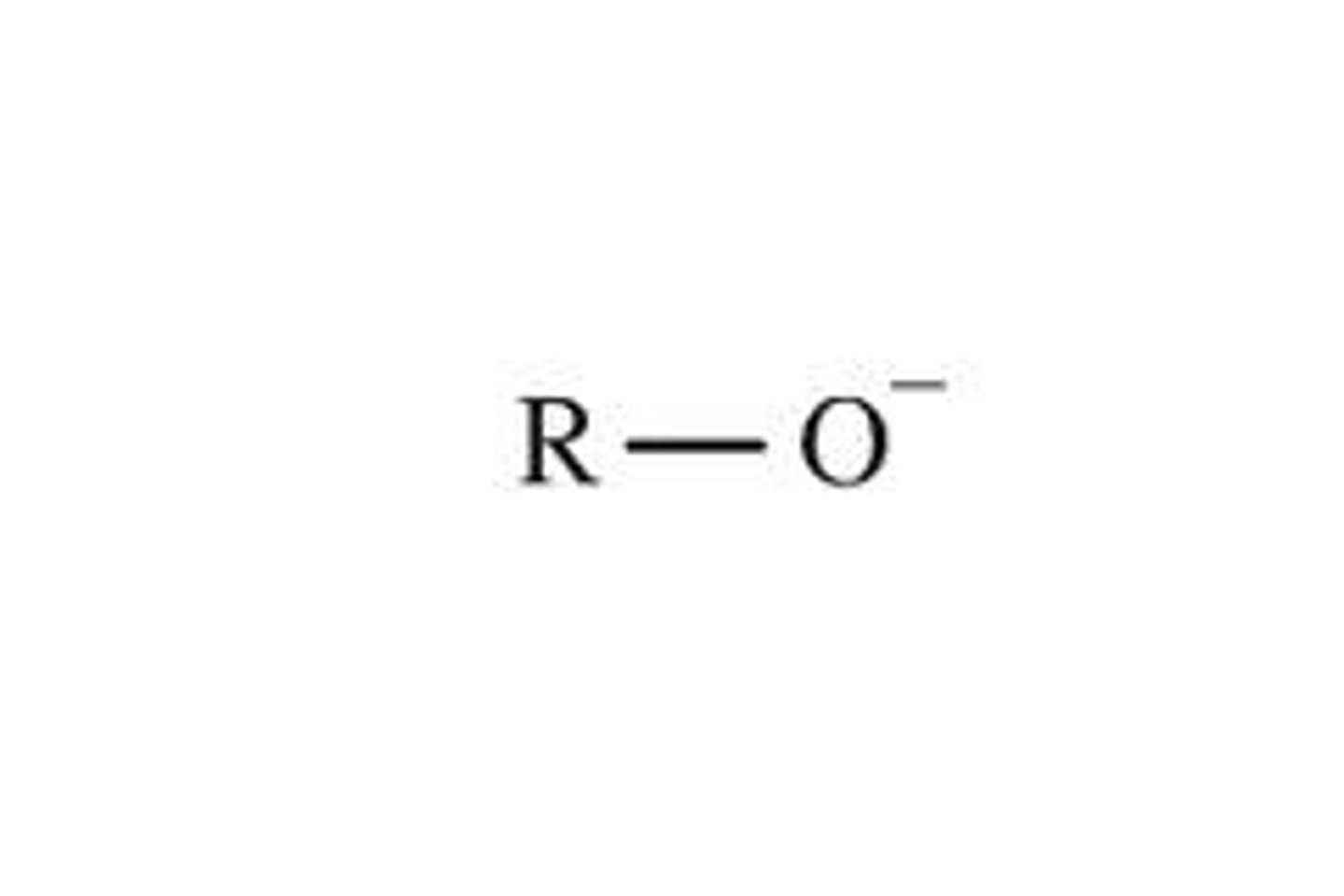
How are alkoxides formed?
1. K or Na -> treating alcohols with alkali metals or treating alcohols with strong bases (NaH or nBuLi)
Alkoxides can be synthesized from any alcohol, which means any alcohol can be used as a nucleophile in substitution chemistry
•
William Ether Synthesis
Alkoxide + Alkyl halide = ether
Three ways you can turn an acohol into an electrophile:
acid catalysis (propenate the OH to form +OH2)
conversion of sulfonate esters
conversion into halides
Conversion of Sulfonate Esters
1.RSO2Cl, pyridine
1 degree or 2 degree alcohols
making the leaving group in an alcohol (OH) a better leaving group by adding sulfonyl chloride. This forms esters with GREAT leaving groups: Ts, Tf, Ms the stereochemistry is left the same as it was.
-make sure you know the reaction mechanism
Alcohol conversion into alkyl halides
using acid catalysis with H-X
sn2 (primary alcohols are Sn2)
hydrogen halides to form a alkyl halide , depropenate an H-X and then use the X- to kick off the leaving group
reaction mechanism
Alcohol conversion into alkyl halide (PBR3)
1. PBr3
you can make an alkyl halide which contains the Br3
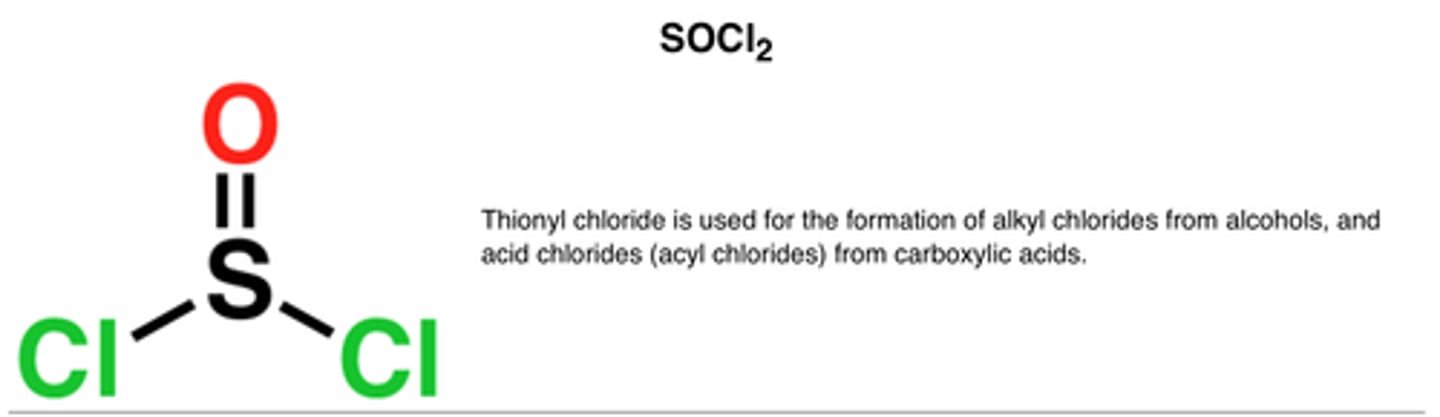
Alcohol conversion into alkyl halide (SoCl2)
SOCl2
1. SOCl2, pyridine
alcohol is the nucleophile, SOCl2 (electrophile), forms an alkyl halide
Ether synthesis with acidic conditions
alcohol + alcohol = ether. this only makes symmetrical ethers
-reaction in text book page 508
isolated system
multiple bonds that are separated by one or more saturated carbon atoms
(more than one single bond)
cumulated system
two or more double bonds connected by a common carbon atom (i.e. 1,2-diene); the 𝜋-bonds of each double bond are perpendicular to
each other, making the central carbon sp-hybridized
C=C=C
Conjugation
- a system of connected p-orbitals that are aligned to allow the delocalization of electrons; it is commonly observed in systems with alternating multiple and single bonds, but heteroatoms can also be involved with conjugation
apoxides
this is a ring that is formed from addition of mCPBA to an alkene. Under acidic conditions, strong acid will protonate the epoxide and lead to a partial positive charge developing on the most substituted carbon• Under basic conditions, a good nucleophile will attack the least substituted carbon to open up the epoxide selectively