Physical Science Final
1/228
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
229 Terms
What is the Solid state of matter?
Rigid, retains its shape unless distorted by force

What is the Liquid state of matter?
Flows readily
Conforms to the shape of a container
Has a well defined boundary
Has higher densities than gases

What is the Gas state of matter?
Flows readily
Conforms to the shape of a container
Does not have a well-defined surface
Can be compressed readily

What is the Plasma state of matter?
Has gaseous properties but also conducts electricity
Interacts strongly with magnetic fields
Commonly exists at higher temperatures

What are chemical elements?
Represent the simplest and purest forms of everyday matter. Each element is composed of atoms.
What is the nucleus?
Every atom has a very dense, compact core called the nucleus. It is composed of two kinds of particles:
Protons (+ electric charge)
Neutrons (No electric charge)
The nucleus is surrounded by one or more particles called electrons (- electric charge)
Explain how atoms are distinguished from other atoms.
Each element's atoms have a fixed number of protons.
The number of protons = atomic number.
The atomic number identifies the element.
Example: Helium (He)
Has 2 protons → atomic number = 2
Elements are represented by a chemical symbol (1 or 2 letters).
What are Chemical compounds?
Chemical compounds are made from building blocks called molecules.
Every molecule of a particular compound consists of the same unique combination of two or more atoms.
Each water molecule consists of two hydrogen atoms and one oxygen atom.
What is the behavior of atoms and molecules in solids?
Attractive forces between particles are very strong; the atoms or molecules are rigidly bound to their neighbors and can only vibrate.
Atoms or molecules in a solid arranged in a regular geometric pattern are called crystals.
Solids that do not have a regular crystal structure are called amorphous solids.
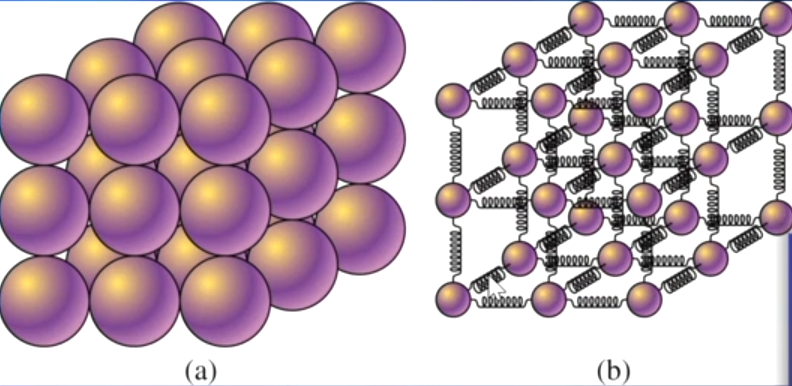
What is the behavior of atoms and molecules in liquids?
The inter-atomic forces are insufficient to bind the atoms rigidly. The atoms are free to move and vibrate.
What is the behavior of atoms and molecules in gases?
Inter-atomic forces are negligible unless atoms are very close.
Moving gas atoms exert force when they hit container walls → creating pressure.
Example: Air molecules hitting a tire create inflation pressure.
No movement = no pressure.
Gases are easily compressed due to the large spaces between atoms.
Sufficient compression brings atoms close together → forms a liquid.
What are Fluids?
A fluid is a gas or a liquid
A gas expands to fill any container
A liquid (at fixed pressure and temperature) has a fixed volume, but deforms to the shape of its container.
The density (p) of any substance is its mass (M) per volume (V)
P = M / V
What is Pressure?
Pressure (P) is the amount of force (F) per unit area (A)
P = F / A
The outward force per unit area that the fluid exerts on its container
Inward force per unit area that the container exerts on the fluid.
What is Atmospheric Pressure?
Come from the weight of the column of air above us.
At sea level, atmospheric pressure is up to 10% lower during hurricanes!
What is Pascal’s Principle?
A change in pressure applied to an enclosed fluid is transmitted undiminished to every point of the fluid and to the walls of the container.
What is Archimede’s Principle?
States that the buoyant (upward) force acting on an object in a fluid at rest is equal to the weight of the fluid displaced by the object.
This means the object will sink until the weight of the object equals the weight of the water displaced by the object.
Density less than fluid = Float (e.g. Wood)
Density greater than fluid = Sink (e.g. Rocks)
What is Bernoulli’s Principle?
In order for the same amount of mass to pass through a cross-section of the wide and narrow sections in a certain amount of time, the fluid must flow faster in the narrow section.
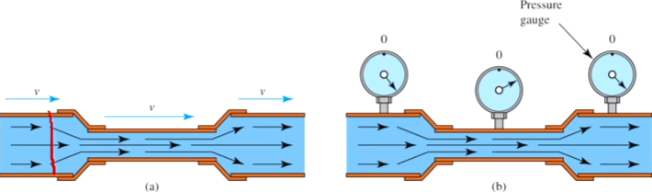
What is Bernoulli’s Equation?
Swiftly moving fluid exert less pressure than do slowly moving fluids
Bernoulli’s equation is a consequence of Conservation of Energy
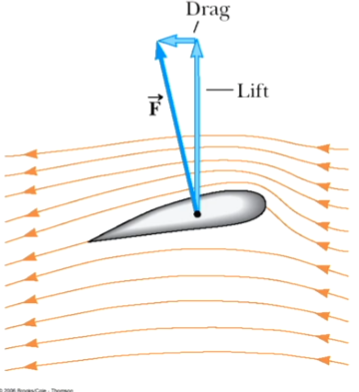
The air speed above the wing is greater than the speed below
The air pressure above the wing is less than the air pressure below
Called Lift
Other factors are also involved
What is the Zero’th Law of Thermodynamics?
Our experience tells us that objects placed in contact will eventually reach the same temperature. We say that they are then in thermal equilibrium. This is the basis for:
The Zero’th Law of Thermodynamics:
If A is in thermal equilibrium with C
And B is in thermal equilibrium with C
Then A and B are in thermal equilibrium with each other
Objects or systems in thermal equilibrium have the same temperature
What is Temperature generally?
Measured in Fahrenheit, Celsius, and Kelvin
Rapidly moving molecules have a high temperature
Slowly moving molecules have a low temperature
What are the temperature scales?
C → K
kelvins = degrees centigrade + 273
C → F
TF= (9/5)TC + 32
F → C
TC = (5/9)(TF – 32)
What is the Boiling Point of Water, Freezing Point of Water, and Absolute Zero in both Celsius and Kelvin?
Celsius
BPoW → 100C
FPoW → 0C
AZ → -273C
Kelvin
BPoW → 373K
FPoW → 273K
AZ → 0K
What is Kelvin temperature?
The Kelvin temperature of matter is proportional to the average kinetic energy of the constituent particles
Kelvin temperature ∝ average kinetic energy of atoms
This helps explain many phenomena that we will examine.
It explains why the pressure of a gas increases as the gas’ temperature
What is the relation between KE and temperature increase?
As the temperature increases, the average KE (speed) of the particles increases.
At higher temperatures, when the atoms collide with the container walls, they impart more momentum and strike with a larger force.
What is Heat?
Heat is the energy flow resulting from a temperature difference.
NOTE: HEAT AND TEMPERATURE ARE NOT THE SAME
What is Joule’s Expirement?
Joule’s Experiment involved a falling weight that turned a paddle wheel inside a container of water.
As the paddle stirred the water, friction increased the water’s temperature.
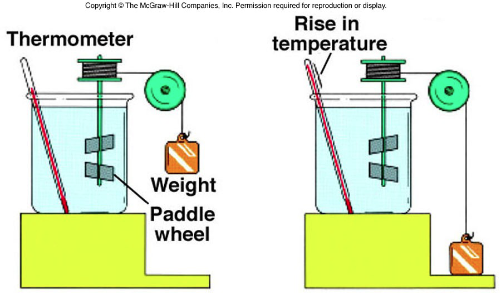
Where did the energy in Joule’s experiment go?
By the First Law of Thermodynamics, the energy we put into the water (either work or heat) cannot be destroyed.
The heat or work added increased the internal energy of the water.
This is the energy stored in the atoms and molecules that make up the water; they move faster.
Describe changing the Temperature of Matter
Some substances are easy to heat (like butter). Some substances are harder to heat (like water).
Different materials ALTER THEIR ENERGY AT DIFFERENT ‘RATES’
Different substances have different capacities for storing internal energy
What is Heat Capacity?
An increase in internal energy causes a rise in the temperature of the medium.
Different mediums require different amounts of energy to produce a given temperature change.
The heat capacity of water is higher than most other substances
What is Specific Heat Capacity?
Materials are characterised by the amount of energy they can store, and how easy or difficult it is to change that energy
Another way to characterise the thermal properties of a material...
How well (or poorly) does a material carry energy from one place to another?
What is Heat Transfer?
Heat transfer is one way of transferring energy to a body
Occurs only when there is a temperature difference between the two bodies (heat flows from hot to cold)
What are the three types of heat transfer?
Conduction
Heat is transferred through a material
(e.g. insulation or glass)
Convection:
Heat is transferred by air or water currents
(e.g. ocean currents)
Radiation:
Heat is transferred when a hot body emits radiation
(e.g. infrared radiation given off by a fire)
What is Conduction?
Heat is conducted across the boundary between two substances.
For a pan on a stove, the conduction occurs because the flame is in contact with the bottom of the pan.
Conduction also occurs within the pan.
The bottom gets hot and makes the top hot.
What is Convection?
Warm air (water) rises, and cool air (water) sinks
Why? Because warm air (water) is less dense and “floats” on cooler air (water)
The rising of warm air (water) creates circulating convection currents
Convection can occur in any fluid
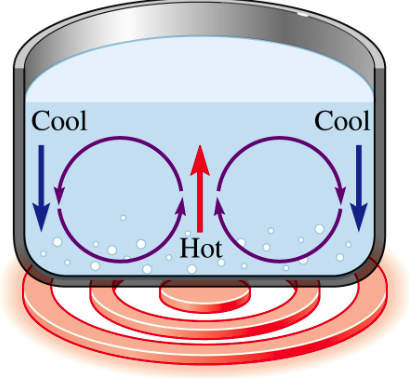
How is convection responsible for weather patterns?
Daytime:
Land heats up faster than water
Warm air over land rises, cool air from over water moves in → Sea breeze
Nighttime:
Water retains heat longer than land
Warm air over water rises, cool air from land moves in → Land breeze
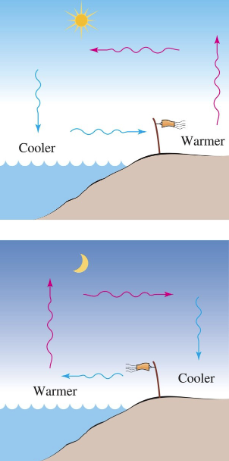
What are examples of Convection?
The sea breeze is caused by differences in temperature between the ocean and the shore
In fact, all weather and ocean currents are caused by convection
A draft in a cold room is caused by convection currents from air leaking through a window or door
A “rolling boil” in a pot is the result of convection
What is Radiation (Heat Transfer)?
Heat is emitted as electromagnetic radiation (e.g. light)
Radiation is emitted at the speed of light
Radiated heat requires no medium (e.g. air) and can propagate through empty space
What is the First Law of Thermodynamics?
Conservation of Energy
In an isolated system the total amount of energy, including heat, is conserved.
The many different kinds of energy are interchangeable.
Although the kind of energy in a given system can change, the total amount cannot
What is the Second Law of Thermodynamics?
Heat flows spontaneously from a hot object to a cold object, but will not flow spontaneously from a cold object to a hot object
It is relatively easy to produce thermal energy by doing work (e.g. against the force of friction).
It is also possible to convert internal (heat) energy to work
What is Entropy and Disorder?
Entropy measures disorder or randomness in a system
In irreversible processes, the entropy of the universe always increases
Local entropy can be reduced by doing work, but total entropy still rises
Energy is conserved, but degrades into its lowest form: heat
If the universe is finite, it will reach uniform temperature (heat death), and energy will no longer be usable for work
What are the unusual properties of liquid water?
Water contracts when heated from
0ºC to 4ºC, then expands when heated from 4ºC to 100ºC.
Just above the freezing point, the coldest (and least dense) water rises to the surface, and lakes freeze from the surface downward.
This unusual property permits aquatic life on Earth to survive winter
What reflects the fact that life requires water?
70% of Earth’s surface is covered by H2O
70% of human body’s weight is H2O
Water can exist in 3 states on Earth’s surface
exists only as solid and vapor on Mars
only vapor on Venus
What is the Atmosphere?
A relatively thin shell of gases surrounding the solid Earth.
Density decreases with increasing altitude
78% Nitrogen, 21% Oxygen, ~1% Other
Briefly describe the history of the development of the Atmosphere
Icy Comet impacts:
Adds H2O to the atmosphere and down to about 70km
Earth cools - water condenses
Lots of rain
Volcanoes add CO2
Most of the CO2 is dissolved in the oceans
Photosynthesising organisms evolve
CO2 + H2O + sun light → glucose + O2
What are the 3 layers of the Atmosphere?
Ionosphere
Stratosphere
Temperature increases with height.
Less turbulent layer.
Troposphere
Describe the Troposphere
Surface to where the temperature stops decreasing with height.
All weather occurs in the troposphere:
“Churning sphere”
Convection causes the “churning”
Temperature decreases with altitude
Describe the Stratosphere
The ozone layer is contained in the stratosphere
No “churning” because of a temperature inversion:
Temperature increases with altitude
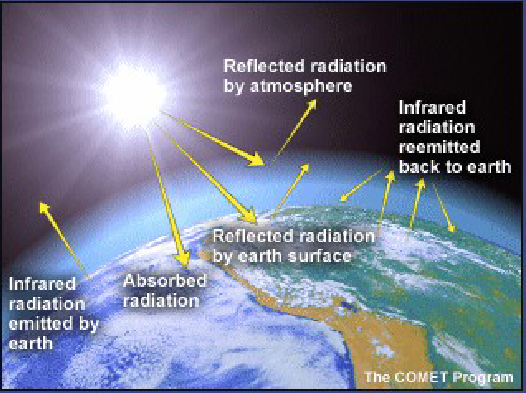
How much of the Earth’s surface absorbs incoming solar radiation?
On average, the earth's surface absorbs only 51% of the incoming solar radiation after it is filtered, absorbed, and reflected.
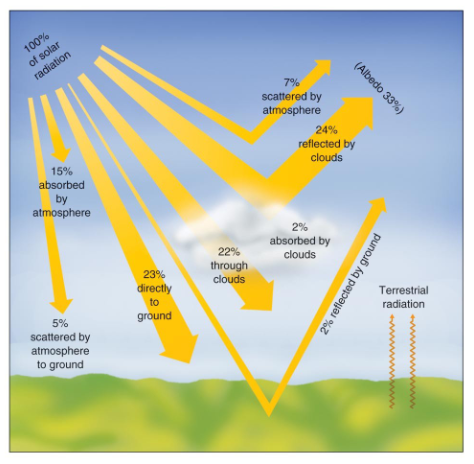
Describe Winds (Global)
The equator receives more direct solar radiation year-round.
Average temperatures are higher at the equator and decrease toward the poles.
This uneven heating drives:
Prevailing wind patterns
High and low pressure areas
Global climate zones
What is the Coriolis Effect?
Caused by an unattached atmosphere over a rotating Earth
The Coriolis Effect is the apparent deflection of moving objects (like air or water) due to the Earth’s rotation.
In the Northern Hemisphere, objects deflect to the right.
In the Southern Hemisphere, objects deflect to the left.
It affects wind and ocean current directions, contributing to global weather patterns.
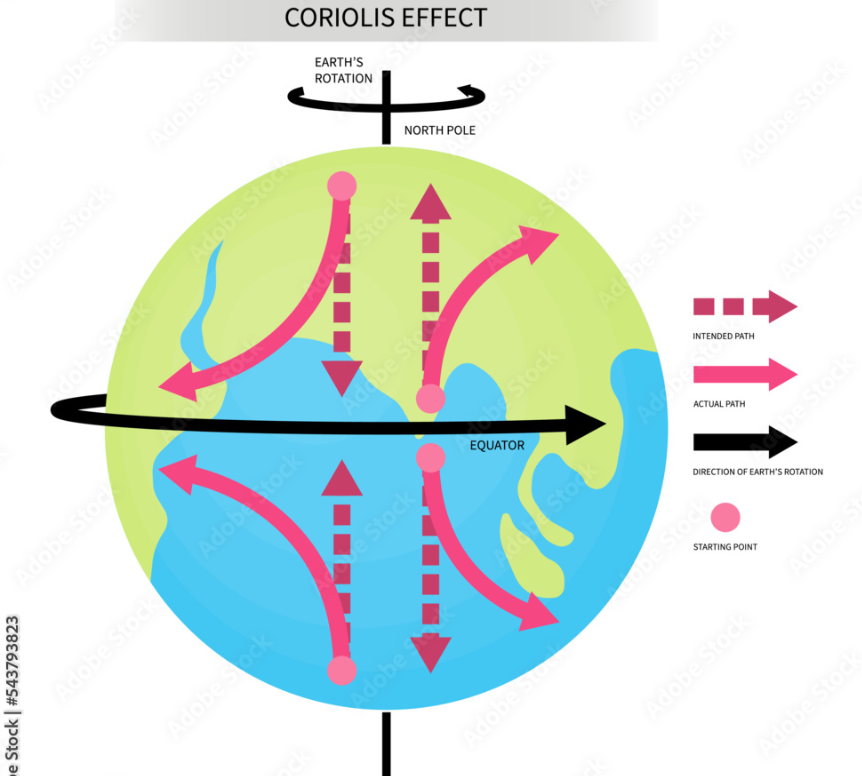
What are Prevailing Winds?
The direction the winds blow most of the time
Sometimes changes due to weather systems
In the middle latitudes (30o- 60o), the prevailing winds are from the west.
These affect climate
Describe Local Wind Patterns
Differential heating creates warm (less dense) and cool (more dense) air.
Cool air sinks and pushes warm air upward → lowers surface pressure.
Rising warm air cools, becomes denser, and sinks → raises surface pressure.
This cycle forms a convective cell of air movement.
Land heats and cools faster than nearby water.
Daytime: The Land is warmer
Warm air over land rises
Cool air from water moves in → Sea breeze
Nighttime: Land cools faster
Air over warmer water rises
Cool air from the land moves in → Land breeze
What is the Cloud-forming Process?
Convection results from differences in temperature
Barriers such as mountain ranges provide lift to air masses
Meeting of moving air masses with different densities:
Warm - less dense
Cold - denser
What are the types of Air Masses?
Continental Polar, “cold and dry”
Originates closer to the Poles over land-locked regions.
Continental Tropical, “warm and dry”
Originates closer to the Tropics over land-locked regions.
Maritime Polar, “cold and damp”
Originates closer to the Poles over water.
Maritime Tropical, “warm and humid”
Originates closer to the Tropics over water.
Arctic, “very cold”
Originates in the very cold
land-locked areas
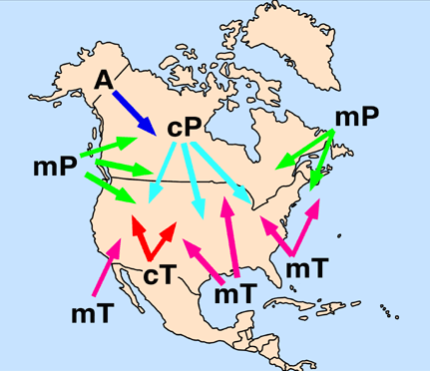
What are Weather Fronts?
A boundary between two different (density) air masses
What is a cold air front?
Cold air advancing and displacing warmer air that exists
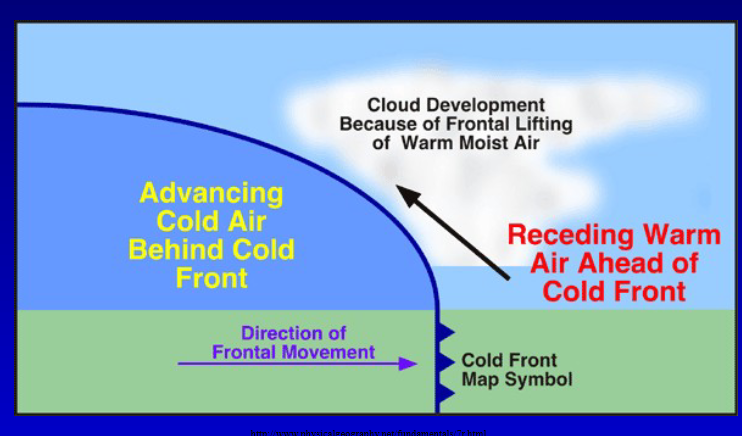
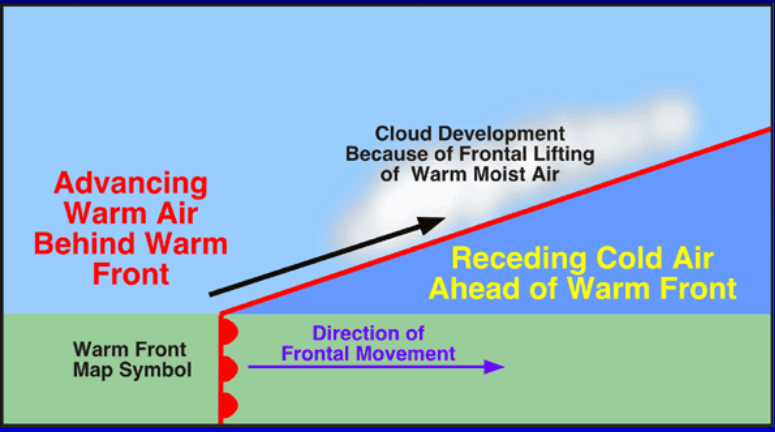
What is a warm air front?
Cold air retreating from an area
What is a stationary air front?
Differing air masses exist along a boundary, but little movement of the air masses
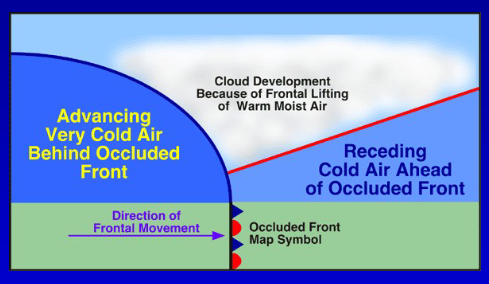
What is an occluded air front?
Complicated process - the surface low becomes surrounded by cooler/cold air
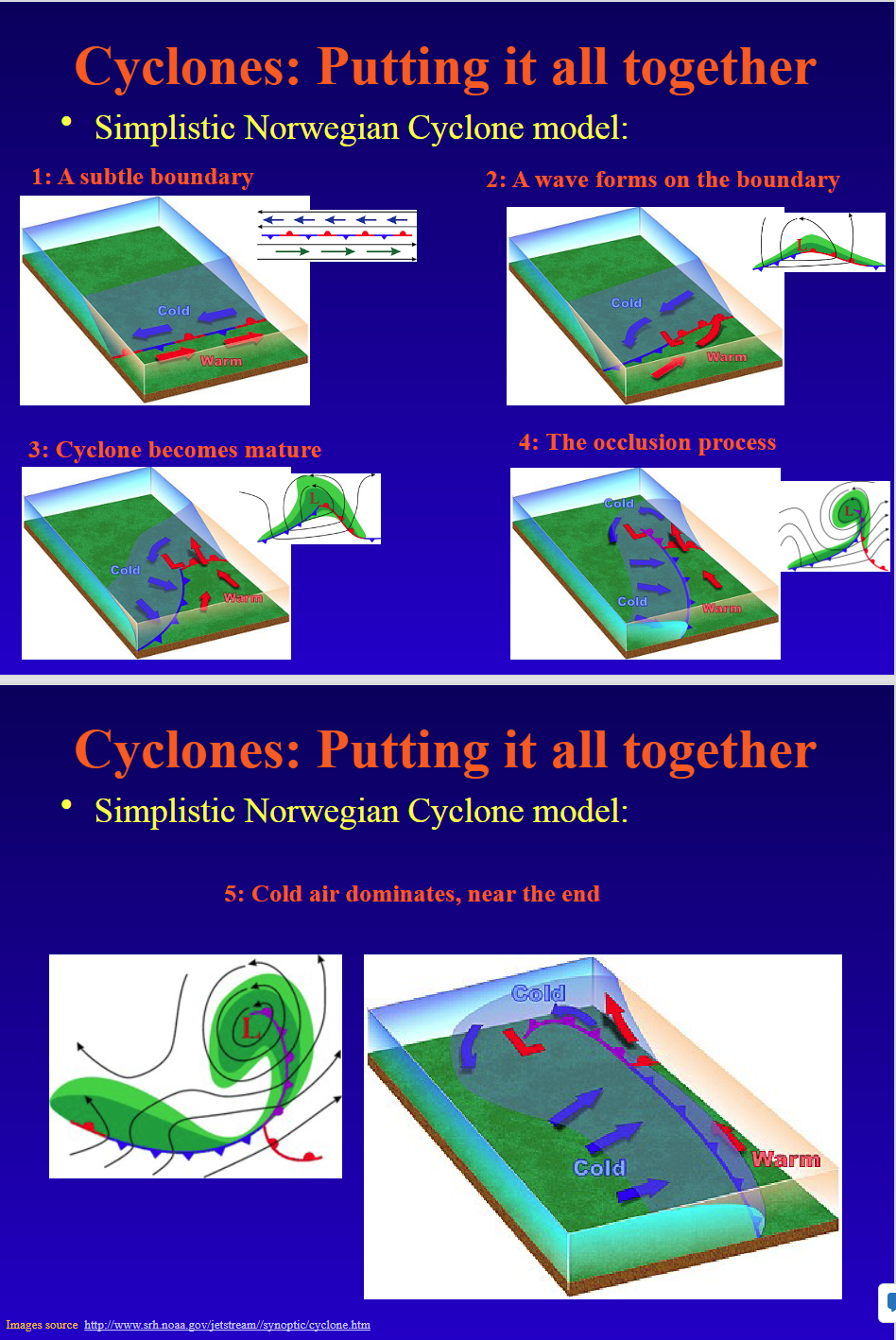
What is the Simplistic Norwegian Cyclone Model?
1: A Subtle Boundary
2: A wave forms on the boundary
3: Cyclone becomes mature
4: The Occlusion Process
5: Cold air dominates near the end
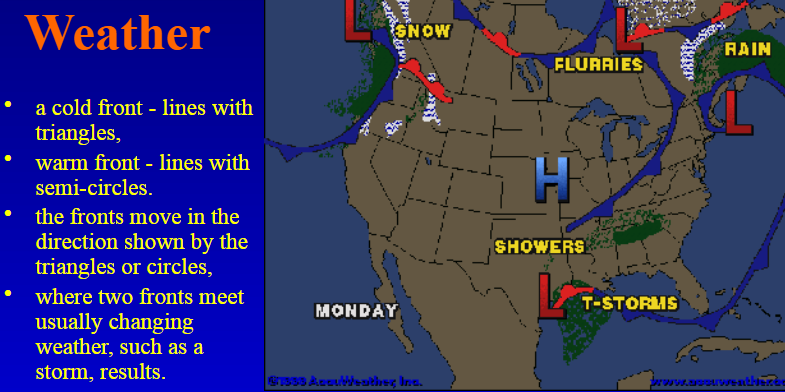
How do fronts affect weather?
Where two air masses meet → causes changing weather (e.g., storms).
High-pressure areas:
Associated with clear skies
Stable weather conditions
Low-pressure areas:
Associated with rising air and cloudy skies
Leads to unstable weather due to increased evaporation and condensation
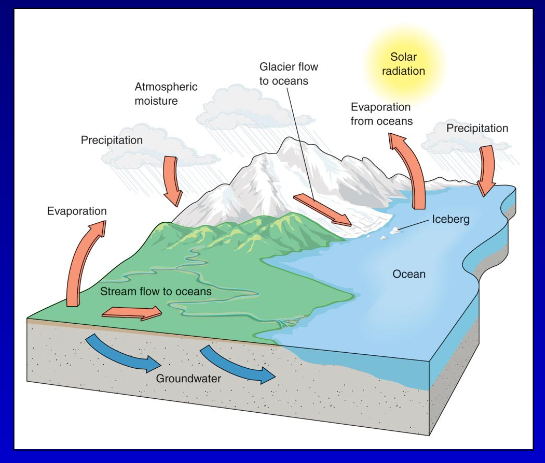
Describe the Hydrologic Cycle
Solar radiation powers the cycle.
Evaporation: Water from land and ocean turns into vapour.
Atmospheric moisture: Water vapour forms clouds.
Precipitation: Water returns to Earth as rain or snow.
Glacier flow: Ice flows downhill into oceans.
Icebergs melt and release water into oceans.
Stream flow: Water flows from land to oceans.
Groundwater: Water moves underground and eventually reaches oceans.
The cycle is continuous, moving water through land, atmosphere, and oceans.
Describe Evaporation
Sun heats up liquid water and changes it to a gas by the process of evaporation
Water that evaporates from Earth’s oceans, lakes, rivers, and moist soil rises up into the atmosphere
Describe the distribution of all the water found on the earth's surface.
Marine (Saltwater)
The ocean contains over 97% of Earth’s water
Brackish (Salt/Fresh)
Found in estuaries, where fresh and salt water meet
Important to aquatic life
The 3rd most productive ecosystem
Fresh Water
Less than 3% of Earth’s water is fresh
75%: Glaciers, unusable by life
24%: Groundwater (incl. soil moisture)
-~1%: Lakes/Rivers
If all Earth’s water fit in a gallon jug, there would be one tablespoon of available fresh water
Describe the unique nature of water on Earth
Water in the atmosphere varies and cycles through the Hydrologic Cycle.
This includes evaporation (water turning to vapour) and condensation (vapour forming clouds), often occurring daily.
Earth is unique in the solar system for having:
Liquid water covers most of its surface
Water vapour in the atmosphere
Frozen and liquid water on land
What are the averages of the hydrologic cycle (regarding evaporation and precipitation)?
On average, more water evaporates from oceans (84%) than returns via precipitation (77%).
More precipitation falls on land (23%) than evaporates from it (16%).
The excess water on land flows back to the oceans through rivers and streams, maintaining balance.
How do differences in densities affect the Hydrologic cycles?
Seawater moves due to density differences, like air does.
Cold water is denser and sinks, while warm water rises.
At the poles, cold water sinks and flows beneath warm water.
It travels toward the equator, warms up, and rises to the surface.
This drives deep ocean circulation (part of the global conveyor belt).
What affects the climate?
Prevailing winds and ocean currents are only two of these
Example: Why is the weather in Paris more pleasant than in Quebec City?
Gulf Stream + Prevailing westerlies
Describe Ice Ages
Ice ages occur over thousands of years.
Explained by Milankovitch cycles:
Changes in Earth’s orbit and tilt affect solar heating.
Precession (wobble of Earth's axis) occurs every 26,000 years.
Currently, the Northern Hemisphere tilts away from the Sun in winter.
In 11,500 years, it will tilt toward the Sun in winter.
Milankovitch suggested ice ages are linked to orbital variations and changes in solar energy reaching Earth.
Describe the constant “changes” in the Earth
Earth (with the Sun) is a closed system — no net gain/loss of matter.
Gradual changes are always happening.
A change in one part affects other parts.
Cycles are connected: water, rock, and atmospheric cycles interact.
Humans have a significant impact on Earth's systems.
What is the Water-rock cycle?
Moving streams of water carry away dissolved materials and sediments as they slowly erode the land.
What is the Ozone hole?
Ozone (O₃) = 3 oxygen atoms; absorbs harmful UV radiation.
Protective cycle:
O₃ + UV → O + O₂
O + O₂ + sunlight → O₃
This ozone layer allowed life to move from oceans to land.
CFCs (chlorofluorocarbons), introduced in the 1950s, damage ozone.
Reaction: 2CO₃ + Cl + sunlight → 3O₂ + Cl
Result:
Ozone destruction → more UV exposure
Risks: skin cancer, crop damage, and ecosystem disruption.
What is Acid Rain?
Caused by pollution from burning fossil fuels.
Nitrogen oxides, sulfur compounds, and hydrocarbons mix with water in the atmosphere → form nitric and sulfuric acids.
These acids fall as acid rain.
Effects:
Damages buildings (e.g., sandstone sculptures)
Kills forests and harms ecosystems
Challenge: No large-scale, commercially viable alternatives to fossil fuels yet.
What are Greenhouse effects and Global Warming?
Like a greenhouse, gases in the atmosphere trap heat → Greenhouse Effect.
Keeps Earth’s average temp at +20°C (vs. -20°C without).
Venus shows an extreme greenhouse effect (≈450°C).
Human activities (mainly burning fossil fuels) add greenhouse gases like CO₂, causing global warming.
CFCs and their alternatives also contribute.
Alternatives to fossil fuels include:
Solar, wind, geothermal, tidal, and nuclear power
Battery-powered vehicles
Earth's temperature naturally varies, but global warming trends appear over long time scales.
Where is the majority of the mass of an atom is located?
In the Nucleus
Proton has ~2000x the mass of an electron
Proton Mass = Neutron Mass
However, electron and proton have equal charges
What is an Electric Charge?
Electric charge does not have continuous values.
An atom’s charge is neutral if it has the same number of protons and electrons.
If it is different, it is ionised.
If protons outnumber electrons = Positive Ion (+ Charge)
If electrons outnumber protons = Negative Ion (- Charge)
How are Ions created?
Electrons move from atom to atom to create ions:
Positively charged ions result from the loss of electrons
Negatively charged ions result from the gain of electrons
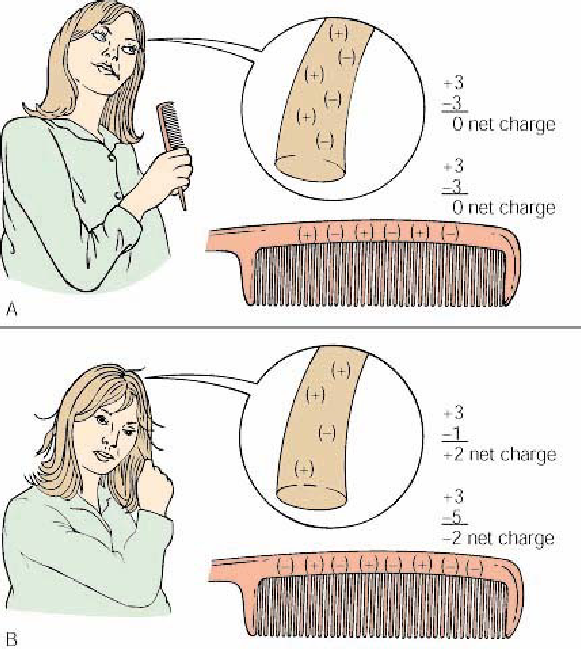
What is Electrostatic Charge?
Comb and hair (A) neutral before combing.
Combing transfers electrons from the hair to the comb by friction, resulting in a negative charge on the comb and a positive charge on the hair
Since every hair has the same charge, they repel each other
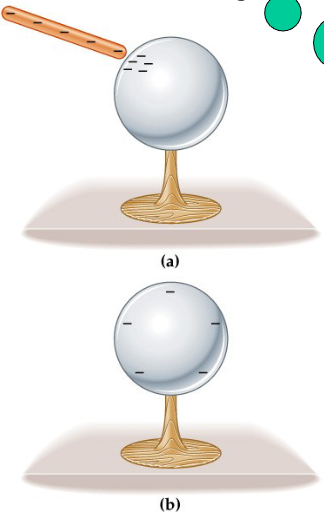
What is Charging by Induction? (Paper and Comb)
The comb has become charged by friction, acquiring an excess of electrons
The paper normally has a random distribution of (+) and (-) charges.
The charged comb is held close to the paper, reorientation of charges because of the repulsion of the charges.
This leaves a net positive charge on the side close to the comb,
Since unlike charges attract, the paper is attracted to the comb
By rubbing a balloon against our hair we can charge it (it has a negative charge), so it can stick to the wall. Why does it happen?
The wall becomes polarised and is attracted to the balloon
This is an example of an insulator polarisation!
What would happen if we charged the balloon positively. Would it still be attracted to the wall or will it be repelled by it? Why does it happen?
It will be attracted
The answer is the same because of the wall’s “opposite” polarisation!
What is Charge distribution on a Conductor?
Since similar charges repel, the charges will be distributed on the surface of the conductor, as far as possible from each other...
The amazing thing is that the inside of a charged conductor will be charge free: shielding
Faraday’s cage (car, airplane)
What is Conservation of Charge?
In isolated systems, charge is conserved
It is not possible to create or destroy a single charge
Charge can be created from energy:
γ-ray = positron+electron
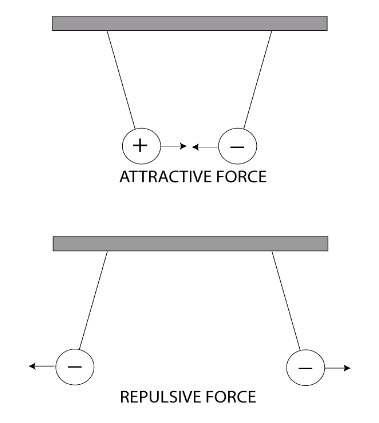
What is Electric Force?
Like Charges Repel, Opposites Attract
Coulomb’s Law
The force between two charged objects is proportional to the product of their charges divided by the square of the distance between them.
Charge is measured in Coulombs, usually called C.
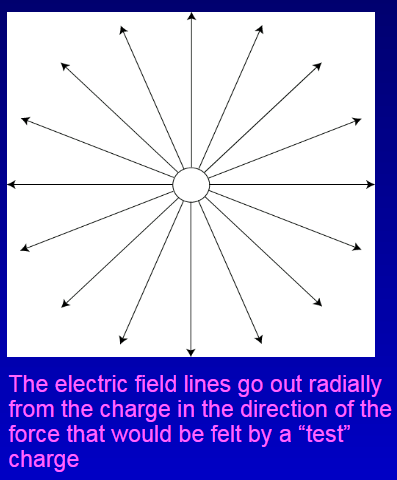
What is The Electric Field?
Any charged object in the vicinity of another charge will feel a force.
Around a charged object, an electric field is present, even if there are no other charges.
The field is responsible for the force.
What are (Electrical) Conductors?
Materials in which electrons can move easily. (Good conductors include metals)
What are (Electrical) non-Conductors?
(Insulators) Electrons do not move easily, tightly bound in atoms (dry air, glass, rubber, plastic)
What is an Electric Current?
Flow of charge
Moving electrons
Electric current means a flow of charge in the same way that a water current flows.
Electrical engineering:
current is opposite of moving electrons (historical reason)!
Unit: Ampere = Coulomb/second
What is an Electric Circuit (Not Current)
Potential = potential energy per 1 coulomb of charge
Electric current requires a potential difference (voltage)
This difference pushes charges through a conductor
Current is maintained by pumping charges to higher potential. Charges then flow back to lower potential, doing work.
Higher voltage = More current
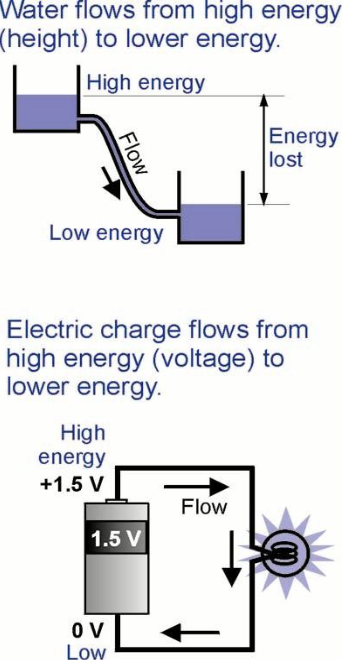
What is the Water analogy for the Electric Current?
Electrical Current: the number of electrons passing through a wire per second
Water current: the number of water molecules passing through the pipe per second
Components:
Pump / Battery are the same
Valve / Breaker is the same
Light Bulb / Nozzle Constriction is the same
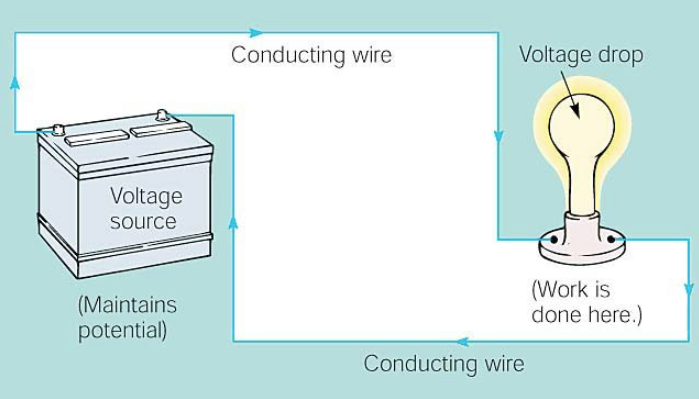
For examples purposes, what is a simple electric circuit?
A voltage source (generator or battery) maintains the electrical potential
Some device (such as a lamp or motor ) where work is done by the potential
Continuous pathways for the current to follow
What is the relationship between resistance and current?
Inverse relationship
High resistance → Low Current
Low resistance → High Current
What is power and energy in electric circuits?
Definition:
Power (Watt) = change in energy (Joule) per unit time (Second)
The rate at which electrical current does work. Watt = Joule / Second
Energy = power X the elapsed time
Electrical energy becomes:
Light (in the light bulb)
Chemical energy
Kinetic energy (of the motor)
Heat (in the oven)
Commercial unit for energy is kilo watt hour (KWh)
How do Thunderclouds and Lightning work?
Collision of water drops – charge separation
Negative charge at the bottom of the cloud induces a positive charge at the surface of the ground
Lightning strikes = electrical charges flow through the ionised air
The potential difference between the cloud and the Earth is several million volts
Moist air becomes conductor
Electrons flow to the Earth
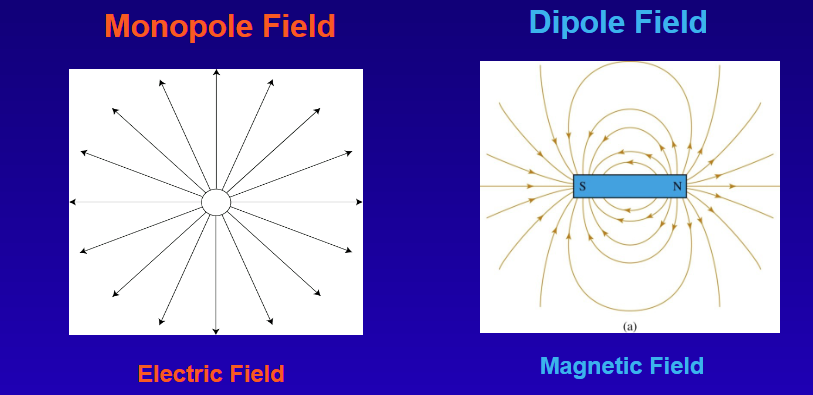
What is Magnetism?
A dipole field can be created with multiple electric charges
Monopole magnetic fields do not exist.
Break a magnet in two:
NO individual north and south poles
Two smaller magnets, each of which has both a north and south pole
It’s impossible to isolate a pole of a magnet
How do Electric Currents produce Magnetic fields?
Hans Oersted discovered that electric current creates a magnetic field.
Noticed a compass needle moved when an electric circuit was turned on.
Magnetic fields form around current-carrying wires.
Unlike electric fields, magnetic fields are not radial—they form circular patterns around wires.
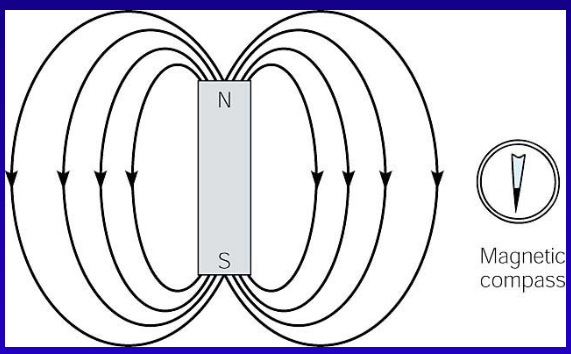
What is the Magnetic Field?
Every magnet has ends, or poles, about which the magnetic properties seem to be concentrated
A magnetic field can be represented by field lines.
Field lines are a map of the magnetic field around a bar magnet.
The needle of a magnetic compass will follow the lines, with the north end showing the direction of the field.
What is the Source of Magnetic Fields?
Electrons in atoms are charges in motion - they produce magnetic fields
In most materials, these magnetic fields cancel out one
another and neutralise the overall magnetic effect
What are Permanent Magnets?
In materials such as iron, cobalt, and nickel, the electrons are oriented in such a ways as to impart magnetic properties to the atomic structure.
These atoms are grouped in a tiny region called the magnetic domain.
What is the Earth’s Magnetic Field?
The Earth’s strong magnetic field is thought to originate from the flowing iron/nickel core
The magnetic axis is tilted about 11.3º from the Earth’s axis of rotation.
The north pole of a compass points to the geographic north pole because it is the magnetic south pole.
The direction of the Earth’s magnetic field flips every few hundred thousand years
The field is currently getting weaker, perhaps in
preparation for another flip