Stage 2 Chemistry, Unit 1-Monitoring the Environment
1/150
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
151 Terms
What are some commons greenhouse gases?
CH4, CO2 H2O, O3, NOx
What is the Greenhouse effect?
A natural process that allows the Earth to trap energy from the Sun through the use of ‘greenhouse gases’ which block a longer wave length of light from being re-emitted into space.
How does the greenhouse effect work?
The Sun emits ultra violet (UV), visible and infra-red (IR) radiation (light). As this light reaches Earth a number of things happen:
30% of the radiation is reflected back to space due to albedo (reflectivity of ice, snow, clouds and water)
22% is absorbed by the gases present in stratosphere
48% is absorbed by the Earth’s surface, and then re-emitted back out to space.
17% of this 48% is then re-radiated as IR radiation (lower in energy, longer wavelengths).
12% of this 17% ‘s thermal re-radiation passes through the atmosphere, back into space
However, the last 5% of the 17% is absorbed by greenhouse gases, which then re-radiates the thermal radiation to other molecules, to space and to the atmosphere
What does the greenhouse effect keep the world temperature at? (meann)
15c
How warm would the world be without the greenhouse effect?(mean)
-17c
What does Albedo mean?
The reflectivity of the world, due to ice, snow, clouds and water.
Structural formula of Water(H2O)
bend(v shape)
Structual formula of Carbon Dioxide
is linear
Structural formula of Methane
tetrahedral
Structural formula of Nitrous oxide
linear (a triple bond and a single bond)
Structural formula of ozone
bent/ v shape (due to electron pair
All greenhouse gases
occur naturally, but have had increased concentration due to anthropogenic influence
What is thermal balance?
the amount of energy entering the atmosphere is equals to the amount emitted
The earth-atmosphere energy balance is achieved as the energy received from the Sun balances the energy lost by the Earth back into space.
What is the enchanced greenhouse effect
Global warming (enhanced greenhouse effect) is the gradual increase in the Earth’s surface temperature over time, caused by an increase in the concentration of greenhouse gases in the atmosphere. The enchanced greenhouse effect is influenced by Anthropogenic activity
What is bad about the enhanced greenhouse effect?
An increase in greenhouse gases results in more IR radiation being absorbed and released around earth, overall increasing the temperature from its maintained temperature.
How much more TW of heat are being absorbed due to the enhanced greenhouse effect?
380TW
What are some examples of anthropogenic influences that have led to global warming?
burning of fossil fuels,
industrialisation (the need for cars and the combustion of their engines)
deforestation
farming of ruminant animals
What are some impacts of the enhanced greenhouse effect?
Global temperature rise
Declining artic sea ice, shrinking ice sheets and retreating glaciers
Rising sea levels
Climate and extreme weather events
Ocean Warming
Carbon dioxide is a
non metal oxide
non metal oxides are
acidic oxides (they react with water to make an acid)
What is the formula for pH?
pH = -log [H3O+]
What is the formula for hydronium concentration?
[H3O+] = 10-pH
What is the formula for hydronium concentration when only hydroxide concentration is known?
1×10^(-14)/OH=[H_3 O]
Ocean acidifacation is a result of
Carbon Dioxide being absorbed by water
Carbon dioxide dissolves in water to form
Carbonic acid ( CO2+H2O→ H2CO3))
Carbonic acid is a
weak acid, meaning it partially ionises to make an equilibrium ( it is a reversible reaction)
What type of polyprotic acid is carbonic acid?
A diprotic acid
Carbonic acid partially ionises to make
Hydronium ions and carbonate ions
H2CO3(aq) + H2O(l) ⇄ H3O+(aq) + HCO3-(aq)
uHydrogen carbonate ions further ionise to form
carbonate ions and hydronium ions
HCO3-(aq) + H2O(l) ⇄ H3O+(aq) + CO32-(aq)
how does the ionisation of Carbonic acid to hydrogen carbonate effect the ocean?
it lowers the pH
How does the addition of more carbon dioxide from global warming disrupt the equilibrium of Carbonic acid?
As more CO2 molecules are introduced to the ocean, they eventually fully ionize to increase the hydronium concentration of the ocean.
(HCO3 +H2O ←→ H+ CO3-2)
Due to le chatlier’s principle, when one side of an equilibrium is increased, another must also increase, as they want to balance out. This leads to more creation of Hydrogen carbonate ions in the ocean.
this also decreases the amount of Carbonate ions present that can be used by crustaceans in the form of CaCO3.
What are the effects of ocean acidifacation?
The increase in hydronium ions in the ocean due to CO2 absorption, due to equilibrium principles, this creates more HCO3- ions and decreases CO3-2 ions.
(HCO3 +H2O ←→ H+ CO3-2)
CO3-2 ions are used in CaCO3 to make shells for coral and marine organisms. so with a decrease of CO3-2 ions, it is harder for their shells to grow.
Moreover, with an increase of H+ ions in the ocean, this attacks the marine organisms’ CaCO3 shells, causing it to break into its individual ions, slowly dissolving its shells.
CaCO3 +2H+ ←→H2O +CO2 +Ca+
what is the formula for the degradation of coral through the addition of a lower pH?
CaCO3(aq) + 2H+(aq)—>CO2(g) + H2O(l) + Ca2+(aq)
this reaction creates more CO2 in the atmosphere which further effects ocean acidifacation.
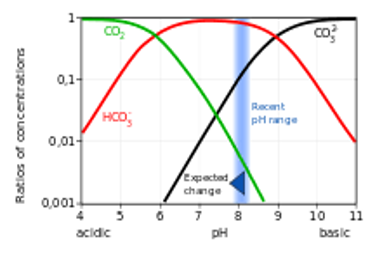
As pH decreases in the ocean, so does the
concentration of carbonate ions. effecting the ocean as marine organisms need carbonate to build their shells
How much percent of the atmosphere does oxygen make up?
21%
How much of the atmosphere does nitrogen make up?
78%
Nitrogen N2 is
a diatomic molecule (with a triple covalent bond)
Nitrogen takes
a large amount of energy to break its triple bond
When nitrogen’s triple bond is broken, it forms
nitric oxide (2NO)
what are some natural causes for the breaking of nitrogen’s triple bond?
Lightning, bushfires, volcanic activity
What are some anthropogenic causes of the breaking of nitrogen’s triple bond?
Internal combustion engines
Jet Engines
Industrial kilns and furnaces
What is the lowest layer of the atmosphere called?
the troposphere
(it is the most effected by human activity
75% of gases present in the atmosphere are found in:
the troposphere
What is photochemical smog?
mixture of pollutants, directly from the source and from secondary reactions, that causes a hazy smog, which can cause respiratory issues and heat inversion
What does photochemical smog contain?
VOC, NOX and O3
What does VOC stand for?
Volitile organic compound
What are the characteristics of a VOC?
VOCs are usually small unburnt organic compounds. becuase they are small, there are present as gases at room temperature, which attributes to their name
How do O3 and NO2 effect photochemical smog?
Nitrogen dioxide gives the smog a brown haze.
What causes the O3 and NO2 in photochemical smog?
Emissions from motor vehicles are the main contributor to photochemical smog’s formation. Incomplete combustion occurs in the enginges, leading to the creation of undesirable products, such as VOCs, Carbon monOxide and carbon.
When emitted from a car, nitrogen reacts with oxygen to form
nitrogen/ nitric oxide( N2 +O2→ 2NO)
When do Nitrogen oxide/Nitric oxide percentages peak during the day?
They peak early in the morning.
After peaking in concentration, Nitric oxide/ nitrogen oxide is further oxidised to form
nitrogen dioxide ( 2NO+O2→ 2NO2 )
NO2 levels peak
After the levels of NO.
When does NO2 dissociate to make an oxygen radical
At noon, as the UV levels are at their highest, as sunlight acts as a catalyst, allowing for the reaction to take place.
During noon, the oxygen radical
combines with an O2 to form trophospheric ozone (O3). This reaction peaks after midday.
What are some effects of photochemical smog for humans and animals?
pollutants can cause eye irritations and respiratory distress.
Eye fluid can react with pollutants to form nitric acid.
while O3 can react to respiratory tissue and reducing the amount of oxygen that can be absorbed in the lungs.
What are some effects of photochemical smog for Plants and Vegetation
Exposure to Ozone present in photochemical smog can cause plant' stomata and leaves to close, limiting the gases exchanged for photosynthesis therefore photosynthesis itself. this distrupts plant growth and makes plants more susceptible to disease.
What are some effects of photochemical smog on industrial systems
Elastic polymers (elastomers) are made brittle by ozone, as ozone breaks the carbon-carbon double bonds in the chains, called degradation, which makes smaller chain molecules, weakening their overall strength due to the decrease in dispersion force (due to size). This also causes weaker interactions between chains.
What are Catalytic converters?
Catalytic converters are present mainly in a vehicles exhaust system, reducing harmful emissions.
What is a catalytic converter made of?
Catalyst support/ substrate, a wash coat and a catalyst.
What is a Substrate (catalyst support) in a three way catalytic converter?
A substratee is usually a ceramic block, which has a honeycomb structure, allowing for a higher surface area for reactions to occur.
What is a Wash coat in a three way catalytic converter?
A wash coat covers the substrate, and further increases the surface area. The wash coat usually contains alumina, as it helps disperse catalytic metals.
What is a catalyst in a three way catalytic converter?
The catalyst is made from materials such as platinum, palladium and rhodium, and causes the harmful chemicals to react into less harmful chemicals.
What does Platinum (Pt) cause in a catalytic converter?
Causes both oxidation and reduction reactions, converting carbon monoxide (CO) into carbon dioxide (CO2) and hydrocarbons (HC) into water (H2O) and CO2
2C8H18+25O2 → 16CO2+18H2O
What does Palladium (Pd) cause in a catalytic converter?
Causes oxidation reactions, to convert hydrocarbons and carbon monoxide into less harmful chemicals.
2CO+ O2 → 2CO2
What does Rhodium (Rh) cause in a catalytic converter?
Causes a reduction reaction, reducing NOx into N2 and O2
2NO+2CO → 2CO2 +N2
Mass =
m (in grams
Volume
V (in litres)
Density (probably dont need this)
p (kg/L)
Avogardo’s number (アボガドロの定数)
NA (6.022×1023) (Mol/L)
Mole
n (Mol) one of avogardo’s number
Molar mass
M (g/mol)
Moles=
n=m/M
Mass concentration =
P= m/v
Molar concentration =
C= n/v
Dilution equation =
C1V1 =C2V2
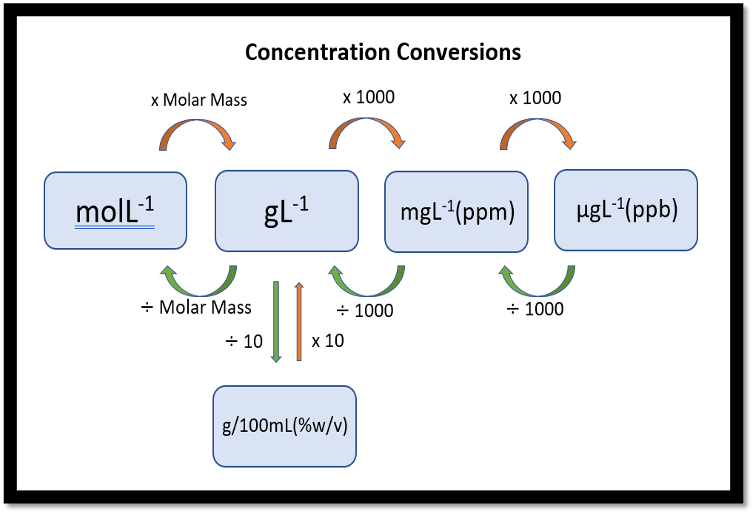
Concentration conversion
Mass-mass Stoichiometry
Use the mass given to convert the chemical into moles. Use the mole ratio to find the moles of the unknown value., when the moles of the unknown chemical is found, find the volume, concentration, or mass
Write fully balanced chemical equation 2. Find the number of moles of known solution (n=c x v) 3. Use mole ratio to find moles 4. Calculate concentration (c = n/v) OR volume (v = n/c) OR Mass (m=M/n)
Limiting reagent
The first reactant that runs out in a reaction. This is determined with comparing the moles of each reactants, and check which is smaller, OR use the mole ratio (coefficients present in the reaction) to determine how much is needed if one is fully used. The mole of the limiting reagent also tells you the mole of the products.
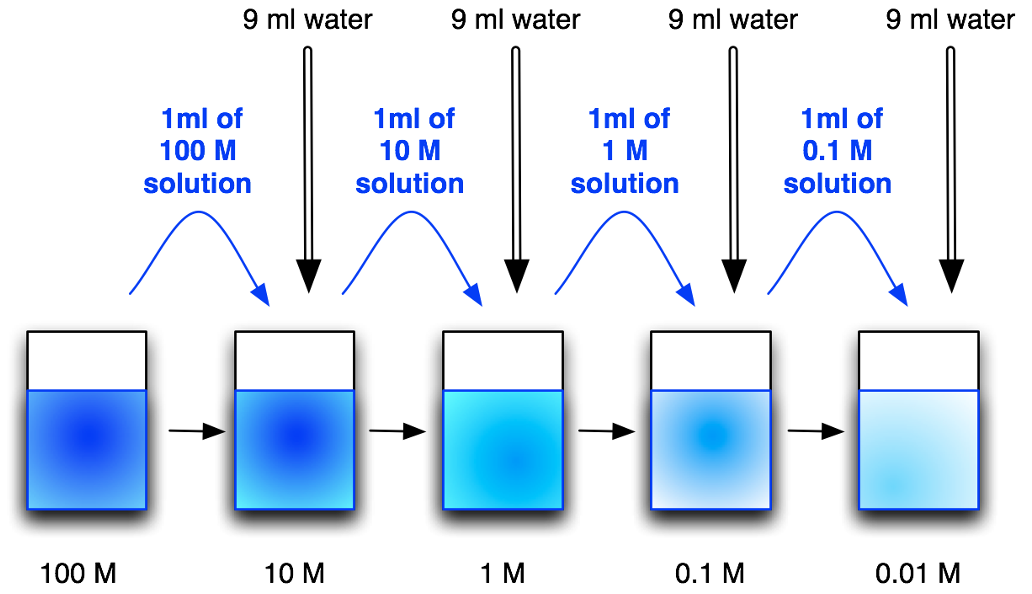
Serial dilutions
are done to generate a series of solutions from a single standard solution, to create calibration graphs.
Standard solution
Clean all glassware
Weight out the mass of solute needed for the solution
Transfer the mass of solute to a clean beaker (use solvent to remove excess solute from the surface)
Dissolve the solute in appropriate amount of solvent
Transfer the solution from the beaker to the volumetric flask
funnel used to ensure all of the solution is transferred.
Add solvent to the volumetric flask through the use of a Burette until the solution is parrallel with the graduation mark at eye level. (bottom of meniscus)
invert several times until substance is fully dissolved.
Accuracy
About the validity of the results given. How close are your answers to the expected answer.
Precision
Is about consistency between the numbers, and the reliability and reproducibility of the data
Systematic errors
(error in the system or procedure)
Poor accuracy,
definite causes,
reproducible (high precision)
Random Error
Poor precision, no specific causes,
not reproducible (could be accurate but fluctuating results
Direct titration
a solution of an unknown concentration is determined by applying the mole ratio of a known concentration via a neutralisation
Titration steps.
Using the given concentration and volume, find moles. use mole ration to multiply or divide the unknown by the ratio. use the new mole to find the concentration (C=N/v
What is a reagent
a chemical added to start or test a chemical reaction, or determine the presence of a specific chemical substance.
What is an Analyte?
the unknown in a titration reaction
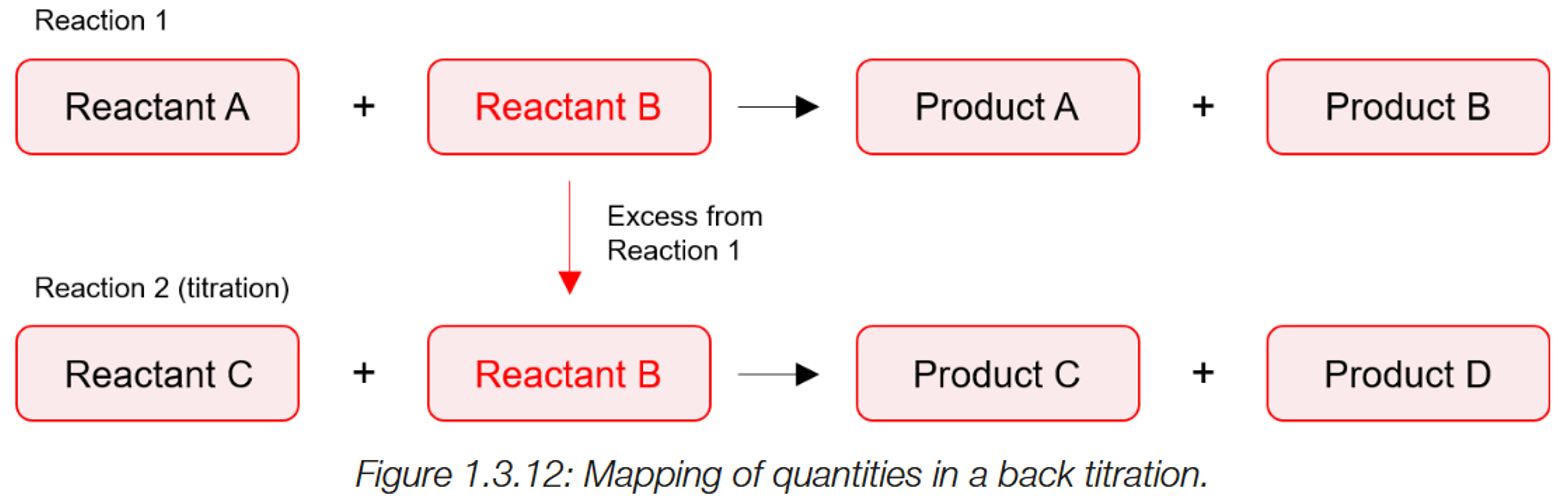
Back titrations
1) A chemical with unknown concentration (analyte) (A) is reacted to a known amount of reagent (B). A+B →
2) However, after this reaction, there is an excess of B leftover. This undergoes a second reaction with another reagent (C). (B+C)
3)The amount of C used to neutralise B’s excess is used to determine how much was of B was leftover from reaction 1 (from neutralising A.) (mole ratio)
4) As the amount of B was added originally is still known, the volume used to neutralised A can be determined
Volume of B to neutralise A (= original volume- excess)
5) The volume of B used for neutralisation is then changed to moles, and via the mole ratio determines the moles of A present.
Why are back titrations and indirect titrations done?
The unknown could be volatile and dissolve into the atmosphere, the unknown could also be a salt, or reacts too slow
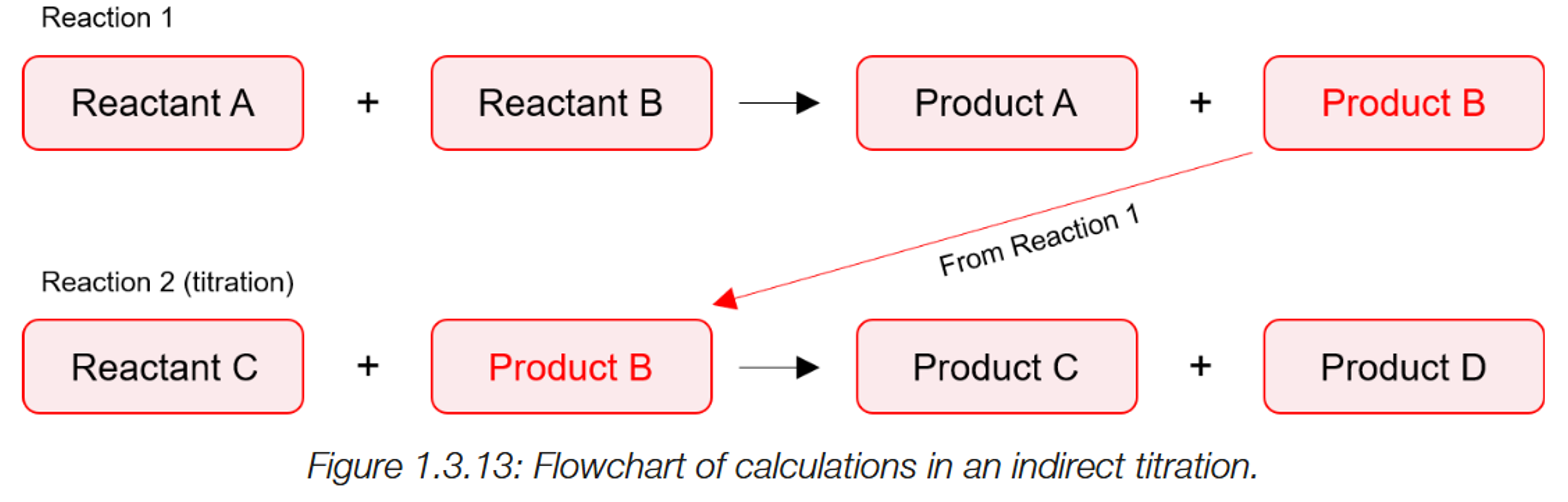
Indirect titrations.
A initial reaction takes place, with two unknowns to make a product (C) (A+B→C).
This product is used in a second reaction. (C+D), where the product is neutralised by chemical D.
The moles of chemical D used in the reaction is found as the concentration is known and the volume used is found.
Using the mole ratio, the moles of C can be found, allowing for reactants A and B from the initial reaction can also be found via the mole ratio.
G/mol→ G/L
xmolar mass
g/L→ ppm
x1000
ppm(mg)→ ppb(µg)
x1000
g/l→ g/100ml
Divide 10
What is Chromatography?
a technique used to isolate and identify individual components of a mixture
What does Chromatography usually contain?
A Stationary and mobile phase
What is a stationary phase?
A solid or liquid medium that components are adsorbed to.
What is a mobile phase?
A liquid or gas that flows through the stationary phase, taking components with it