Topic 17: Transition Metals & their Chemistry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Transition Metals:
d-block elements that form one or more stable ions with incompletely-filled d-orbitals
explain why transition metals have Variable Oxidation States
4s electrons have lower energy than 3d electrons, so they fill before the 3d orbitals. However, once the 3d orbitals start filling, the 4s orbital becomes higher in energy, so its electrons are removed first.
explain why transition metals produce colors
color is due to splitting of 3d orbital; by ligands so different energy difference between d-orbitals lead to different frequencies of light being absorbed and reflected
Ligand
molecule that forms coordinate bond with a central transition metal ion
Complex ion
central metal ion surrounded by ligands
Describe Octahedral Shape
6 ligands, and 6 donated electron pairs
90º bond angle
ex. [Mn(H2O)6]2+ , [Fe(H2O)4(OH)2] , [Cr(OH)6]3-
Describe Tetrahedral Shape
ex. only [Cu(Cl)4]2-
Describe Linear Shape
ex. only tollen’s reagent [H3N → Ag ← NH3]+
Square Planar Shape
ex. cis-platin used in cancer treatment
[2NH3 → Pt ← 2Cl]
Colors of Vanadium Oxidation States
VO2 + / VO3 - (5+) yellow
VO2+ (4+) blue
V3+ green
V2+ purple
Colors of Chromium Oxidation States
Cr2O7 2- (6+) orange
Cr3+ green
Cr2+ blue
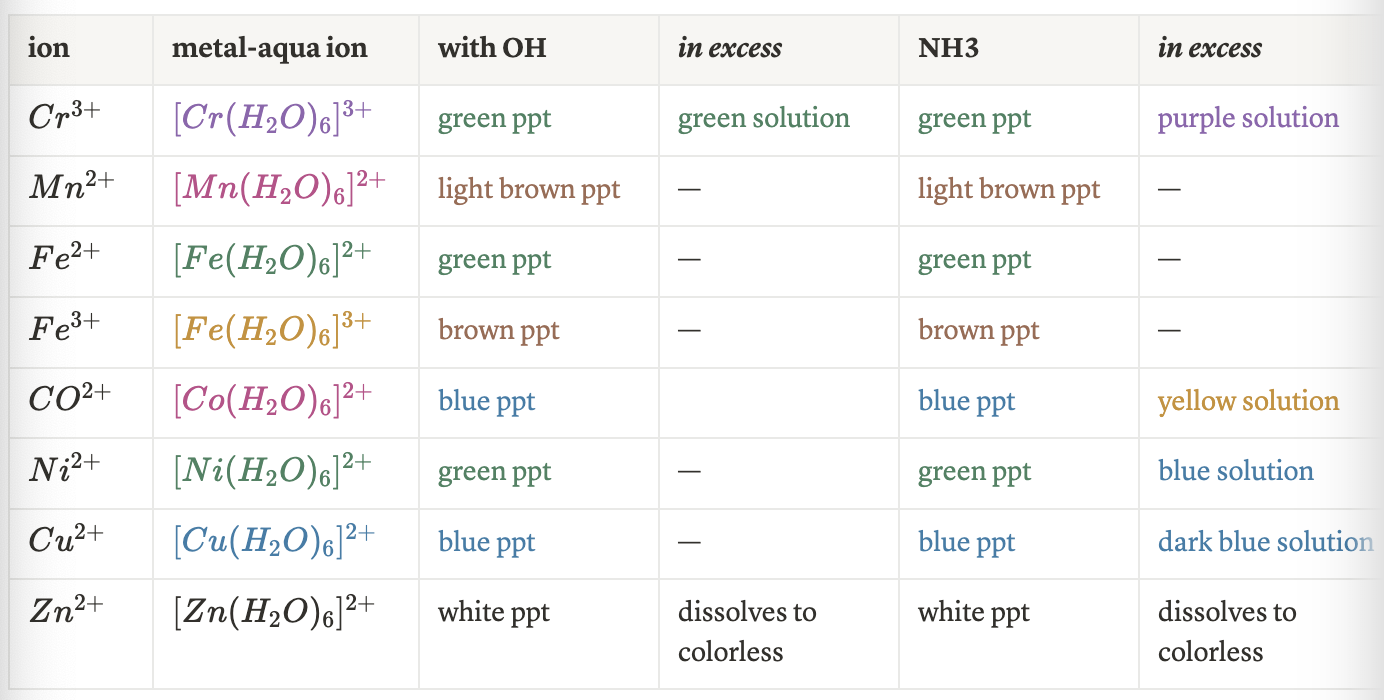
hexaaquachromium + NaOH
intermediate: [Cr(H2O)3(OH)3]
end product: [Cr(OH)6]3-
hexaaquachromium + NH3
intermediate: [Cr(H2O)3(OH)3]
end product: [Cr(NH3)6]3+
hexaaquachromium + H2O2/OH-
intermediate CrO4 2-
end product: Cr2O7 2-
Reactions of Transition Metals