Chemistry 2.6- Reactions of Ions in Aqueous Solution
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
What metal-aqua ions are formed in aqueous solution?
[Cu(H2O)6] 2+
[Fe(H2O)6] 2+
[Fe(H2O)6] 3+
[Al(H2O)6] 3+
Why are some metal-aqua ions more acidic than others?
[M(H2O)6] 3+ complexes are more acidic than [M(H2O)6] 2+ complexes
This is because 3+ ions are smaller and so have a higher charge density, meaning they can attract the water ligands more closely
This weakens the O-H bonds resulting in more dissociation, producing a more acidic solution
How are some metal hydroxides amphoteric?
Aluminium hydroxide, [Al(OH)3(H2O)3], is an amphoteric compound because it reacts with both acids and bases
With HCl:
[Al(OH)3(H2O)3] + 3HCl → [Al(H2O)6] 3+ + 3Cl-
With NaOH:
[Al(OH)3(H2O)3] + NaOH → [Al(H2O)2(OH)4]- + Na+ + H2O
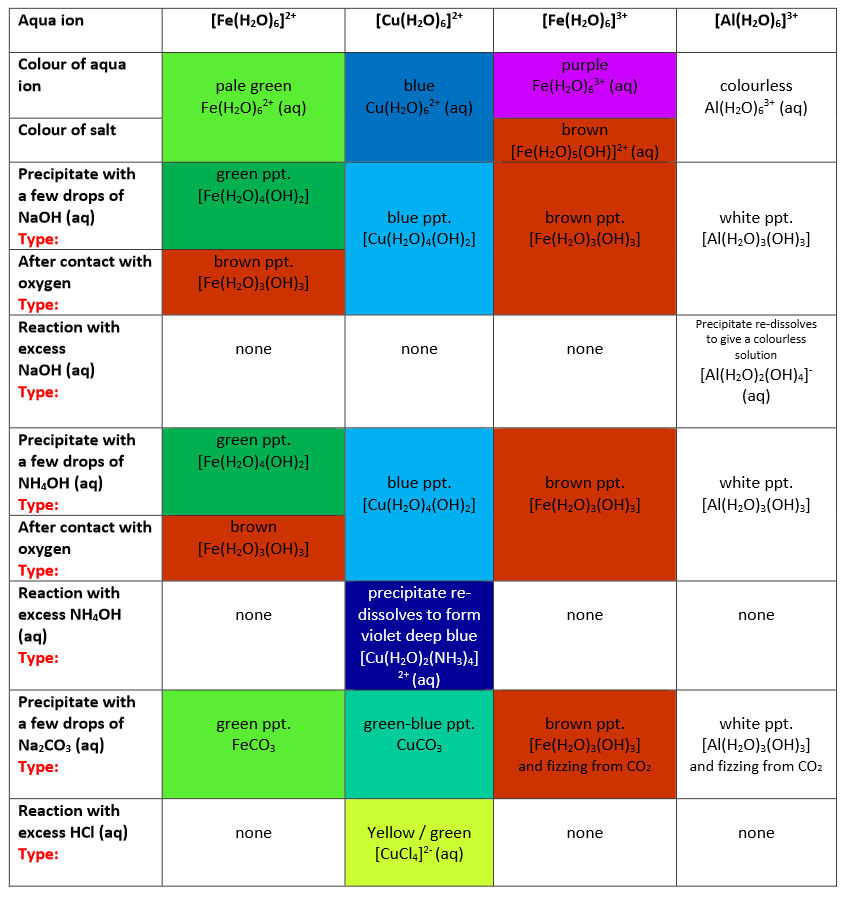
What are the reactions and colours of the metal aqua-ions with OH-, NH3, CO32-, and HCl (including excess)?