PDTI E2
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
In the 3 compartment model, Xps and Xpd stand for what?
Xps- peripheral shallow (ie muscle)
Xpd- peripheral deep (ie plasma)
C = what for IV bolus injection?
C = Dose/Vd e^(-ke * t)
C= Co e^(-ke * t)
The higher the dose, the ____ the Vd, _____the _____ the Co
The higher the dose, the smaller the Vd, the higher the Co
Co=Cmax= dose/Vd
What does e^(-ke * t) represent?
Describes how fast drug disappears. The larger ke the faster the change from 1 to 0
Cl = what in terms of Ke and Vd?
Cl= ke * Vd
What is half life in terms of Cl?
t1/2 = (0.693 * Vd)/CL
ON SHEET
CL = ke * Vd
The larger the CL the _____ the ke and the ____ the half life
The larger the CL the larger the ke and the shorter the half life
CL = ke * Vd
The larger Vd, the ____ the Ke, the ___ the half life
The larger Vd, the smaller the Ke, the longer the half life
What are the units for ke?
1/h or 1/min
What are the units of CL?
L/h or ml/min
What are the units of Vd?
L
What are the units of T1/2?
h or min
How does a different Vd affect half life of the same drug at different concentrations?
it doesn’t
What is AUC in terms of dose?
AUC =Dose/Cl ON SHEET
Is AUC independent or dependent of dose?
dependent
The larger the dose, the _____the AUC
larger
What is the formula for dM/dt?
dM/dt = kmet * X - (kem * M)
amount of metabolite/time= formation of metabolite (kmet) * amount of drug (x) - (rate of drug that is being eliminated * amount of metabolite available to be eliminated)
What happens when the formation of the metabolite (kmet) is larger than the rate of metabolite that is being eliminated (kem)
Since metabolite is formed faster than it is eliminated, metabolite is eliminated more slowly. It is ELIMINATION- Limited
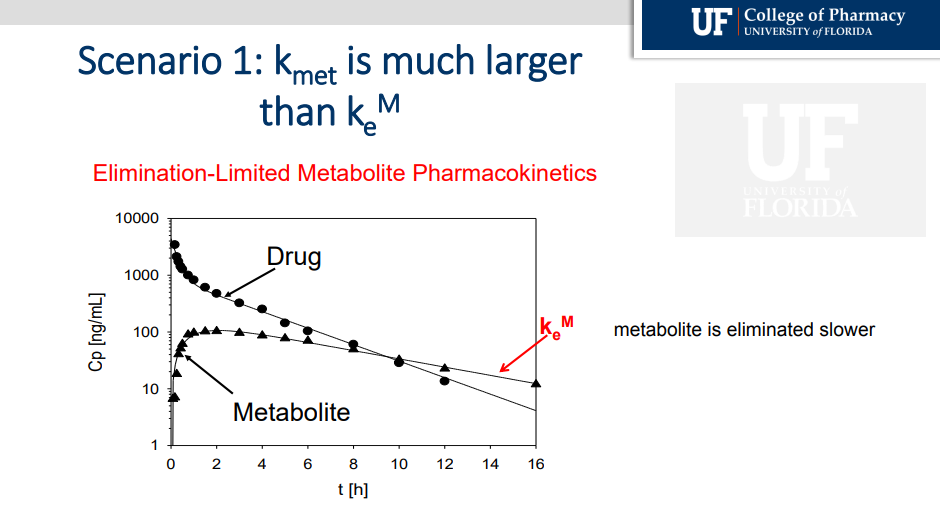
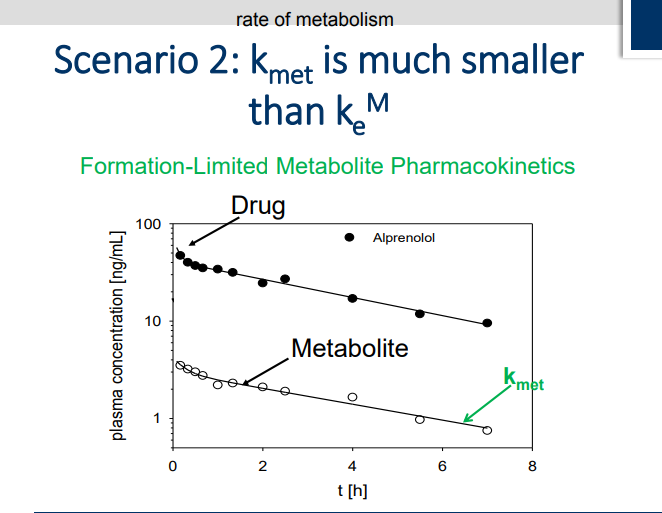
What happens when Kmet is smaller that Kem?
Clearanace of metabolite is determined by formation of the metabolite. Metabolite is cleared quicker
What effect will a 25% decrease in dose have on the PK parameters of a drug whose concentrationtime profile can be characterized by a onecompartment body model (assume linear kinetics)?
cl
vd
t1/2
AUC
Cmax
CL, Vd and T1/2 will stay the same. They are not affected by dose
AUC and Cmax will decrease by 25%
Cmax= D/Vd AUC=D/CL
Drug X was administered as an i.v. bolus at a dose of 1,000 mg. Its therapeutic window is approximately 5-40mg/L. Two plasma samples are drawn for monitoring: one at 2 hours (33mg/L) and one at 12 hours (8mg/L) after dosing. Please compute clearance, volume of distribution and half-life of drug X (assume a 1-compartment body model and linear kinetics).
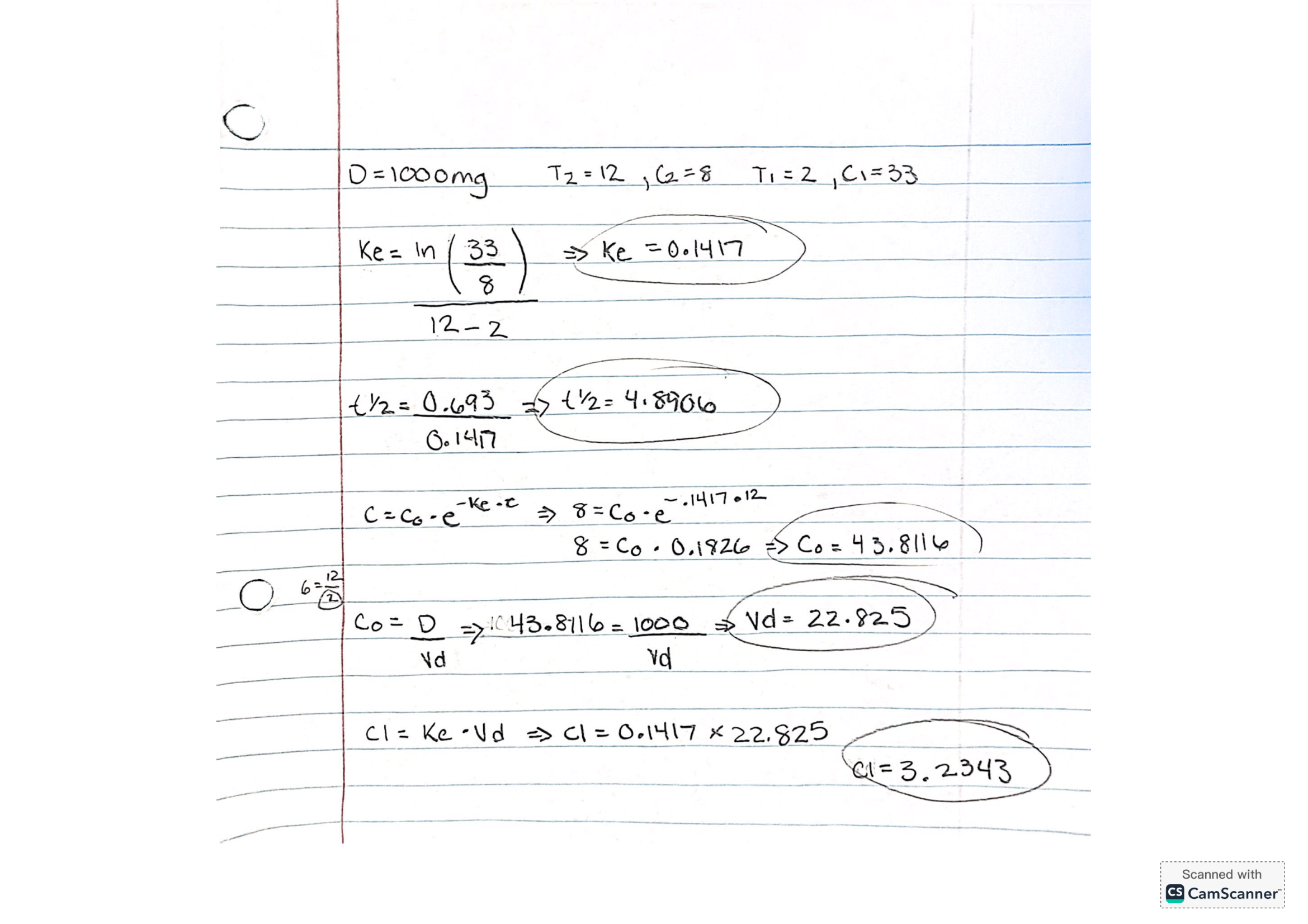
Drug A was administered to a patient as a single IV bolus at a dose of 250mg. Clearance and volume of distribution were determined as 5.7L/h and 25L, respectively. Assuming that the concentration-time profile of the drug can be characterized by a one-compartment body model (assume also linear kinetics), please compute t1/2 , C0 , AUC0-∞ and concentration 5 hours after dosing
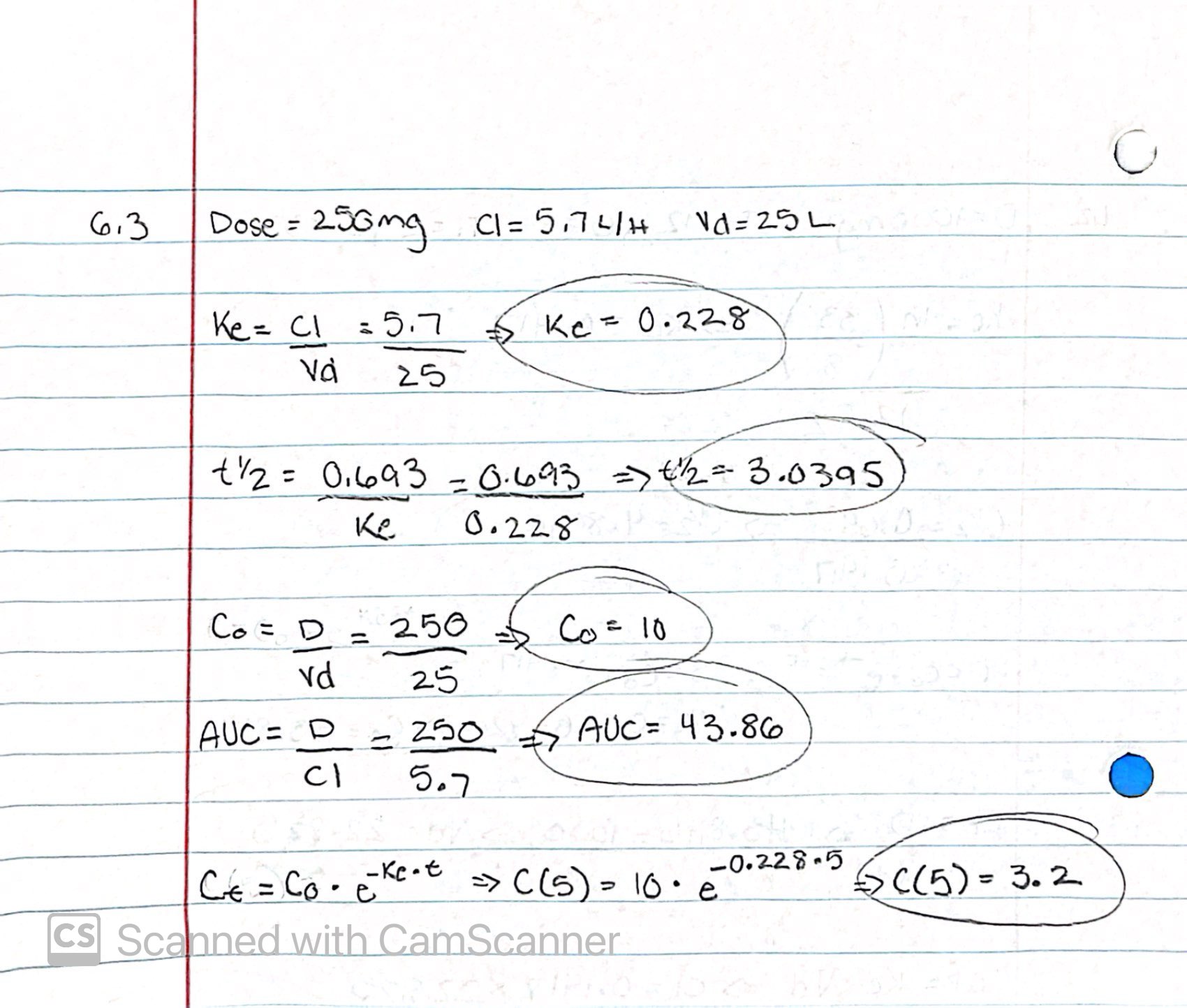
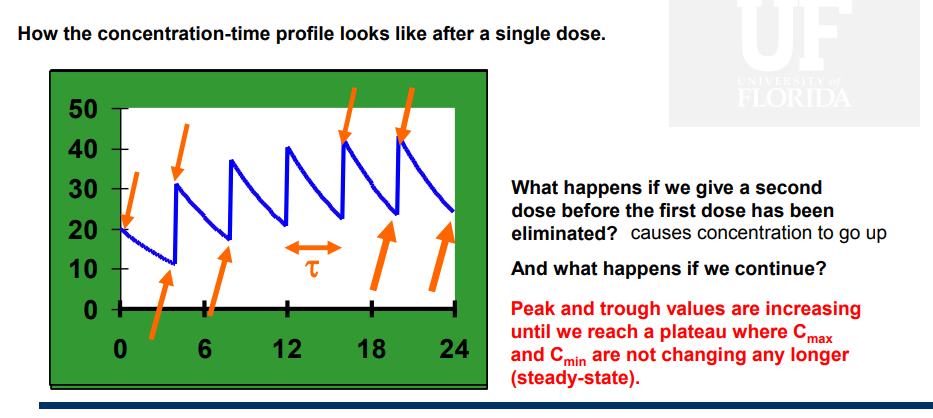
What are Cmax and Cmin?
The point where peaks and troughs reach a plateu
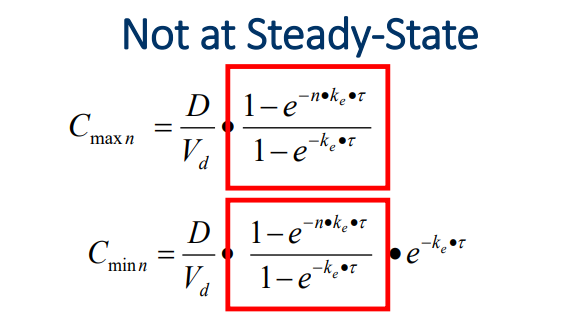
What are the formulas for Cmax and Cmin after the FIRST DOSE
Cmax1= D/Vd
Cmin 1= Co e^ (-ke * tau)
REMEMBER THIS
What is tau?
when the next dose will be given
What is the accumulation factor?
Describes how much higher the concentrations are after n doses compared to those after the first dose
What are the formulas for Cmax and Cmin as they approach the steady state?
Cmaxn= D/Vd ● 1/ 1- e^ (-ke ●tau)
Cmixn= D/Vd ● 1/ 1- e^ (-ke ●tau) ● e^(-ke ●tau)
Will a drug with a bigger or smaller half life have a larger accumulation factor (rss)
The drug with the longer t1/2 (smaller ke value)
rss= 1/ 1 − 𝑒^ −𝑘𝑒●tau
What has a larger accumulation rate? A drug with a tau of 2 or 4?
2, Smaller tau = larger rss. More frequent dosing
Amount of drug in the body=
Cp *Vd
When is the steady state reached?
Amount of drug entering the body= Amount of drug leaving the body
How long does it take to reach the steady state?
about 5 half lives
How can you reach the steady state faster?
By using a loading dose. Loading dose = Dose*rss
What is the maximum concentration after 15 IV bolus injections of 800mg every 6 hours? Please assume that the volume of distribution is 20L and that the first-order elimination rate constant equals 0.5h-1 .

C bar= Cavg=
AUC/tau = Dose/ (CL●tau) ON SHEET
AUC is a cumulative expression that averages concentrations over time
What is C bar?
The drug concentration where the drug elimination rate is equivalent to drug intake rate
dose not depend on Vd
What is fluctuation?
Cmax/Cmin
What is the formula for F, fluctuation?
F= e^ (ke ● tau)
The larger the Ke or the larger the tau, the _____ the F
larger
What is the formula for tau?
tau = lnF / ke
The elimination half-life of an antibiotic is 3 hours with an apparent volume of distribution of 20 % of body weight (200 mL/kg). The usual therapeutic range for this antibiotic is between 5 and 15 µg/ml. Calculate a dosing regimen (multiple IV doses) that will just maintain the serum drug concentrations between 5 and 15 µg/mL for a 70 kg male

Bioavailability (F)
The fraction of an administered dose of a drug that reaches the systemic circulation in its active form
Flip flop kinetics
A pharmacokinetic phenomenon that occurs when the rate of absorption (𝑘𝑎) of a drug is slower than its rate of elimination (𝑘𝑒 ). Ka is terminal phase, not ke
Ka < Ke
Formulation type in flipflop kinetics
modifed release formulations with/ drugs with slow absorption. Longer Tmax
C avg is used to assess
whether drug levels remain within the therapeutic window over time
Absolute BA
when the systemic availability of a drug administered extravascularly e.g., orally, is compared to its intravenous administration
Relative BA
when the systemic availability of a drug administered orally, is compared with that of oral standard of same drug
Bioequivalent
rate and extent of absorption of the test drug do not show a significant difference from those of the reference drug when administered at the same molar dose under similar experimental conditions
For BE studies test and reference product must contain:
Same active pharmaceutical ingredient, In the same dosage strength, In similar dosage form (e.g., immediate/controlled release), Same route of administration
If the half-life is long what study should you do?
parallel design. Requires larger sample size
Highly variable drug should have what design study?
replicate design
What is the ideal study?
2 period cross over design
Accepted bioequivalence range
80%/125% rule
Two-compartment Model using Macro-constants
𝐶 𝑡 = 𝐴 ∙ 𝑒 −𝛼∙𝑡 + 𝐵 ∙ 𝑒 −𝛽∙t
a= distribution phase’ b= elimination phase
In three compartment models, what are the macroconstants?
a= initial distribution
b= intermediate distribution phase
y= elimination phase
Which PK parameters are calculated to evaluate the bioequivalence studies?
AUC and Cmax are the pivotal parameters for bioequivalence determination