Thermochemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Define enthalpy change?
name given to amount of heat given out/absorbed in a reaction carried out at constant pressure
Exothermic
negative delta h values
Endothermic
positive delta h values
Standard Conditions for temperature
298 K
Standard conditions for pressure
1 atm
Define enthalpy change of formation
Enthalpy change when 1 mol of products is formed from its constiutent elements in their standard states under standard conditions
You must end up with 1 mol of the compound so
fractions on LHS
Enthalpy change of combustion
Enthalpy change when 1 mol of a substance is combusted in excess oxygen under standard conditions
You must end up with:
1 mol of what you’re burning
In __________ reactions, the heat transferred to _______________ can be measured by carrying out the reaction in an ___________ container: ______________
chemical
surroundings
insulated
calorimeter
Equation for q
mc∆t
equation for ∆H
-mc∆t / n
How can we go from J to KJ
x100
Define bond enthalpy
the enthalpy needed to break 1 mol if the bond to give separated atoms in a gaseous state
Define average bond enthalpy
average value of the enthalpy required to break a given type of covalent bond in the molecules of a gaseous species
∆H
Bonds Broken - Bonds Maked
Define Hess’ Law
Total enthalpy change for a reaction is independent of the route taken from the reactants to the products.
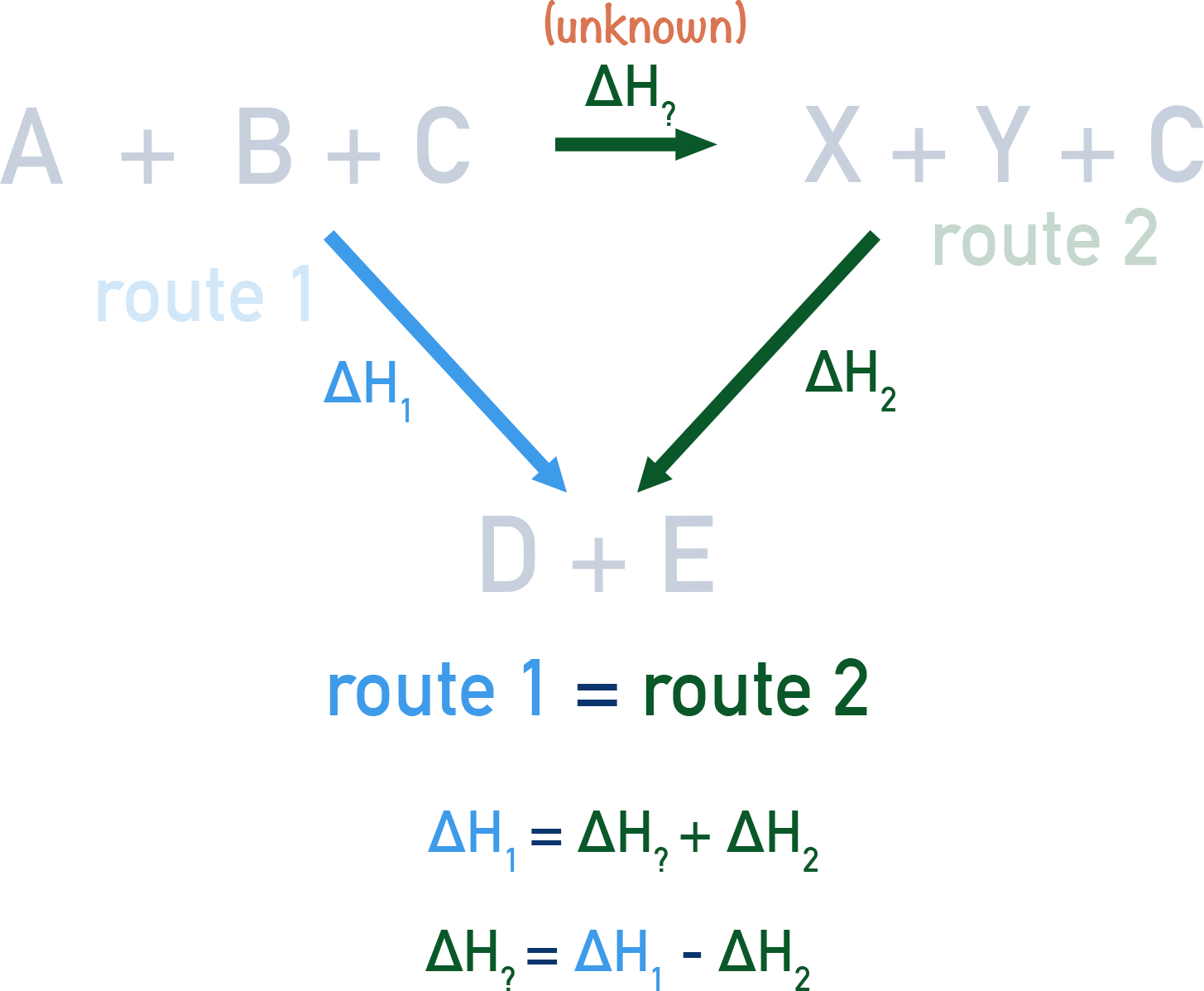
Combustion Hess Cycle
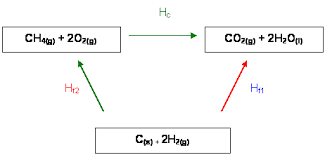
Formation Hess Cycle
How can we find total ∆H using hess cycles
Sum of anti clockwise arrows - Sum of clockwise arrows