Plant Physiology Ch. 6 Solute Transport | Quizlet
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
Molecular and ionic movement from one location to another is known as..
transport
The spontaneous "downhill" movement of molecules is termed...
passive transport
What happens in passive transport at equilibrium?
no further net movements of solutes can occur without the application of a driving force
The movement of substances against a gradient of chemical potential, or "uphill," is termed...
active transport
Is active transport spontaneous or not spontaneous?
not spontaneous. it requires that work be done on the system by the application of cellular energy
- can accomplish this task by coupling transport to the hydrolysis of ATP
Biological transport can be driven by four major forces:
concentration, hydrostatic pressure, gravity, and electric fields
The _________________ for any solute is defined as the sum of the concentration, electrical, and hydrostatic potentials
chemical potential
What is the importance of the concept of chemical potential?
It sums all the forces that may act on a molecule to drive net transport
Neutral solutes have ________________ influenced by _______________
chemical potential; mass-action potential
Charged solutes have both a chemical potential and a charge component =
electrochemical potential
__________________ is a voltage across a membrane that influences the distribution of charges solutes across a membrane
membrane potential
µj (chemical potential) =
µj (chemical potential of j under standard conditions) + RTlnCj (concentration component) + zjFE (electrical potential component) + VjP (hydrostatic pressure component)
µjA > µjB means..
passive transport occurs spontaneously down a chemical-potential gradient
µjA = µjB means..
at equilibrium. If there is no active transport, steady state occurs
µjA < µjB means..
active transport occurs against a chemical-potential gradient
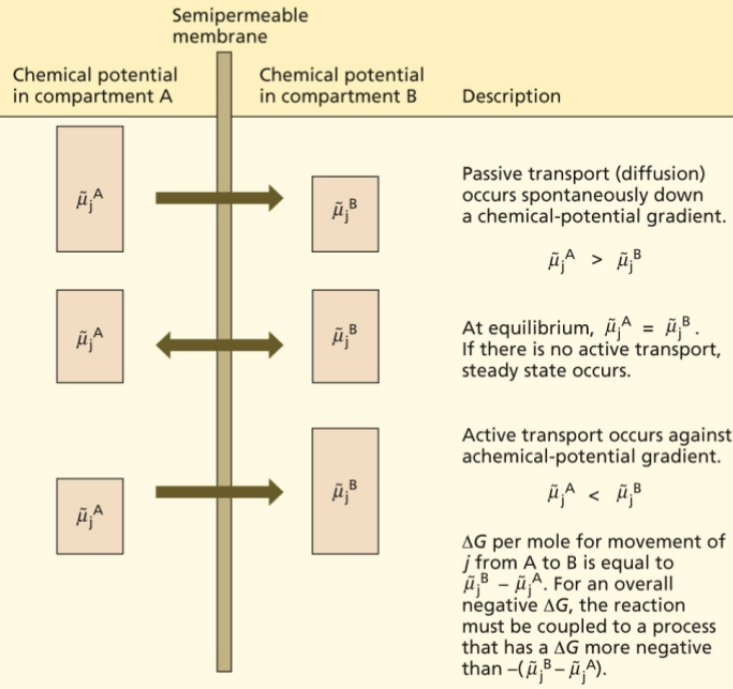
ΔG per mole for movement of j from A to B is equal to..
µjB - µjA
For an overall negative ΔG, the reaction must be coupled to a process that has a ΔG more negative than...
-(µjB - µjA)
Diffusion (passive transport) always moves molecules energetically downhill from areas of...
higher chemical potential to areas of lower chemical potential
Movement against a chemical-potential gradient is indicative of..
active transport
µsi =
µs* + RTlnCsi
What is the equation fo the chemical potential of sucrose inside a cell?
µs^i = µs* + RTlnCs^i
What is the equation for the chemical potential of sucrose outside the cell?
µs^o = µs* + RTlnCs^o
What is the equation for the difference in the chemical potential of sucrose between the solutions inside and outside the cell?
RTln([S]c/[S]o)
If the sucrose different in chemical potential is negative, then...
sucrose can diffuse inward spontaneously
A postive +Δµs =
energy must be applied to move solute into the cytosol
A negative -Δµs =
passive transport into the cell
Ions have ___________ and ____________ potential
electrical and chemical
- electrochemical potential
What is the equation for electrochemical potential and what do the components mean?
µI = µ^oI + RT*ln[I] + zFE
µ^oI = standard electrochemical potential of I (ion) at 1 M
z = valence of the ion (K+ = +1, Mg2 = +2, Cl- = -1, etc.)
F = Faraday constant (96,500 coulombsmol-1) (coulomb = JV-1)
E = electrical potential of the medium in volts (V)
What is the equation for the electrochemical potential difference?
ΔµI = (µI)c - (µI)o = RTln([I]c/ [I]o) + zFDE
ΔE = Eo - Ec =
electrochemical potential difference (volts) between two aqueous media separated by a membrane
A membrane with a charge across it is..
polarized
How can an ion move passively against its concentration gradient?
if the appropriate voltage (electric field) is applied between two compartments
The extent to which a membrane permits the movement of a substance is called the..
membrane permeability
When salts diffuse across a membrane, an _______________ can develop
electrical membrane potential (voltage)
A potential that develops as a result of diffusion is called a..
diffusion potential
Membrane potentials collapse (=0mv) as charge gradients reach..
0
If the concentration of potassium chloride is higher in compartment A ([KClA > [KCL]B), ptassium and chloride ions will diffuse into..
compartment B
If the membrane is more permeable to potassium ions than to chloride ions, potassium ions will diffuse _________ than chloride ions, and a charge separation will develop, resulting in establishment of a ________________
faster; diffusion potential
When concentrations don't collapse, what happens?
membrane potential is maintained
Membranes affect _________, but not. ___________
rate; final equilibrium
What does the Nernst equation state?
that at equilibrium, the difference in concentration of an ion between two compartments is balanced by the voltage difference between the compartments
An ion at equilibrium will have the same _________________ inside and out
electrochemical potential
What is the Nernst equation?
ΔE = (2.3RT/zF)(log[Io/Ic])
The Nernst equation can be further simplified for a univalent cation at 25C..
ΔE = 59 mV (log[Io/Ic]).
A membrane potential of -59 mV will "hold" a ____________ concentration gradient across the membrane for a monovalent cation
tenfold
The Nernst equation can be used at any time to determine..
whether a given ion is at equilibrium across a membrane
- distinction must be made between equilibrium and steady state
______________ is the condition in which influx and efflux of a given solute are equal, and therefore the ion concentrations are constant over time
steady state
A modified version of the Nernst equation, the ________________, includes all permeant ions and therefore gives a more accurate value for the diffusion potential
Goldman equation
_________________ is a major determinant of the membrane potential
Proton transport
Any active transport mechanism that results in the movement of a net electric charge will tend to move the membrane potential away from the value predicted by the Goldman equation. Such transport mechanisms are called..
electrogenic pumps
- common in living cells
The membrane potential of plant cells have two components:
a diffusion potential and a component resulting from electrogenic ion transport
The term transport proteins encompasses three main categories:
channels, carriers, and pumps
What is the proton motive force (PMF)?
the free energy stored in an electrochemical for H+, expressed in mV
What is permeable to small non-polar molecules?
pure lipid bilayer
biological membranes are also permeable to...
ionic, polar, and larger molecules
Proteins facilitate _________ and _________ movement of solute across membranes
active and passive
_____________ are transmembrane proteins that function as selective pores through which ions, and in some cases neutral molecules, can diffuse through the membrane
Channels
Transport through channels is always ____________, and the specificity of transport depends on ______________ and _________ more than on _______________
passive; pore size; electric charge; selective binding
As long as the channel pore is open, substances that can penetrate the pore diffuse through it extremely..
rapidly
- channel pores are not open all of the time
Channel proteins contain particular regions called ________ that open and close the pore in response to signals
gates
Signals that can regulate channel activity include..
membrane potential changes, ligands, hormones, light, and posttranslational modification as phosphorylation
____________ specialize in the transport of specific inorganic ions as well as other organic metabolites
Carriers
Carrier proteins are..
facilitators mostly for organics and some ions, very selective, substrate binds to protein, conformational change in protein, slower (100s-1000s molecules/second)
Channel proteins are...
'pores' through the membrane that transport mostly ions, selective (e.g. K+ >> Na+), no direct interaction between solute and protein, very fast (~108 ions/second!), regulated by "gating" (either opened or closed)
_____________ open or close in response to membrane potential:
Inward rectifying K+ channels open when the membrane is hyperpolarized and let K+ in; outward rectifying K+ channels open when the membrane potential is low and let K+ out
Voltage gated channels
Passive transport via a carrier is sometimes called ____________, although it resembles diffusion only in that it transports substances down their gradient of electrochemical potential, without an additional input of energy
facilitated diffusion
To carry out active transport, what must happen?
a carrier must couple the energetically uphill transport of a solute with another, energy-releasing event so that the overall free-energy change is negative
__________________ is coupled directly to a source of energy other than Δµj, such as ATP hydrolysis, an oxidation-reduction reaction, or the absorption of light by the carrier protein
Primary active transport
Membrane proteins that carry out primary active transport are called..
pumps
Most pumps transport..
inorganic ions
____________________ refers to ion transport involving the net movement of charge across the membrane
electrogenic transport
____________________ involves no net movement of charge
electroneutral transport
The ____________ generates the gradient of electrochemical potential of H+ across the plasma membrane
plasma membrane H+ -ATPase
the ____________ and the _____________ electrogenically pump protons into the lumen of the vacuole and the Golgi cisternae
vacuolar H+ -ATPase; H+-pyrophosphatase
________________ uses the proton gradient created by primary active transport (the proton motive force)
secondary active transport
Secondary active transport is driven indirectly by..
pumps
There are two types of secondary active transport:
symport and antiport
What is symport?
the two substances move in the same direction through the membrane
What is antiport?
coupled transport in which the energetically downhill movement of one solute drives the active transport of another solute in the opposite direction
Which of the following best describes the chemical potential of a neutral solute across a membrane?
ΔGx = 2.303RT*log([X]c/[X]o]
Which of the following can contribute to the membrane potential of a plant cell?
- Negatively charged macromolecules (proteins, nucleic acids) being held inside the cell
- P-type ATPases pumping protons out of the cell at the expense of ATP
- Cations moving out of a cell down a concentration gradient
ΔG = RT*ln([I]c/[I]o) + zFΔE is the electrochemical potential difference of an ion. Based on this equation, name at least three variables that contribute to the distribution of the ion?
- valence of the ion(K+=1, Cl-=-1, Mg2+=2)
- electrical potential of medium(volts)
-concentration inside/outside of cell