Amine and Amide
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
Amine
Are derivatives of ammonia NH3.
Amine
Contain N attached to one or more alkyl or aromatic groups.
Tri-n-octylamine
Metal extractants
2-ethylhexylamine
Additives for metal detergent
Dibenylamine
Cutting fluid additives
Primary amine
one carbon group is bonded to the nitrogen atom.
Secondary amine
two carbon group is bonded to the nitrogen atom.
Tertiary amine
three carbon group is bonded to the nitrogen atom.
Methyl amine
What is this structure called?
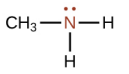
Dimethyl amine
What is this structure called?
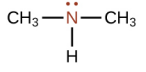
Trimethyl amine
What is this structure called?
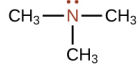
Amine
Boiling point is higher than alkanes
Amine
Boiling point is lower than alcohols of similar mass
Hydrogen bond (Polar N-H Bond)
This provides hydrogen bonding in 1° and 2° amines, but not 3°
1-5 carbon atoms
Amines are soluble in water they have______
N atom
hydrogen bonds
polar O-H bond
water
Amines are soluble in water because____ in smaller amines forms with the in __
Bronsted-lowry base amines
attract a H+ from H2O to the N atom.
Amines
This react as bases
Weak bases
Amines in bronsted-lowry bases are _____ in water
Bronsted-lowry bases (amines)
What is the reaction in the picture?

Bronsted-lowry bases (amines)
What is the reaction in the picture?

Amine salts
Neutralization forms
Amine salt
is named by replacing the amine part of the name with ammonium followed by the name of the negative ion
Neutralization
What reaction is in this picture?

Amides
an amino group(–NH2) replaces the –OH group of carboxylic acids.
Amide
are classified according to the number of carbon atoms bonded to the nitrogen atom.
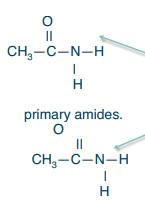
Primary amide
What structure is in this picture?
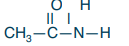
Secondary amide
What is the structure?
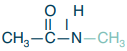
Tertiary amide
What is the structure?
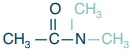
Hydrogen bonding of amide
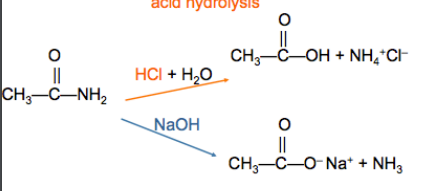
Hydrolysis of amide
Chemical and Physical properties of Amide
Hydrogen bonding of amide
What reaction is happining in the picture?
Hydrolysis of amide
What reaction is in this picture?