Ch-2: Is Matter Around us Pure? (NCERT-Class-9)
0.0(0)
0.0(0)
Card Sorting
1/81
Last updated 3:09 PM on 11/14/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
1
New cards
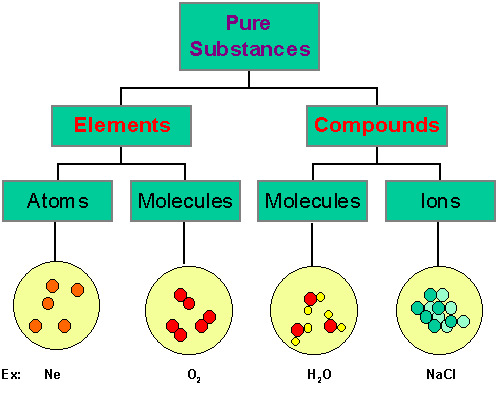
Pure substance
A substance that consists of a single type of particles.
2
New cards
Characteristics of a pure substance.
Constituent particles of a pure substance are the same in their chemical nature.
3
New cards
Can a substance be separated into other kinds of matter by any physical process?
No
4
New cards
Mixture
A substance that contains more than one substance.

5
New cards
What are the characteristics of a mixture?
The characteristics of a mixture are:
1. It has variable composition.
2. shows the properties of the constituent substances.
3. constituent particles of a mixture are different in their chemical nature.
1. It has variable composition.
2. shows the properties of the constituent substances.
3. constituent particles of a mixture are different in their chemical nature.
6
New cards
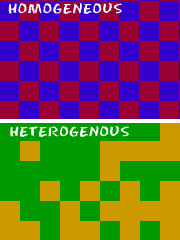
What are the types of mixtures?
homogeneous and heterogeneous.
7
New cards
homogeneous mixture
A mixture that has a uniform composition throughout.
8
New cards
heterogeneous mixture
A mixture that contains physically distinct parts and have non-uniform compositions.
9
New cards
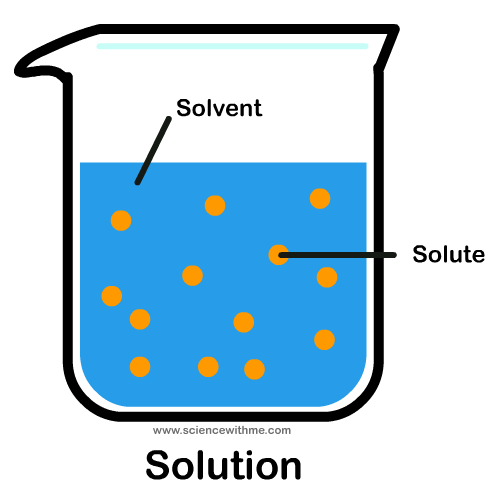
Solution
a homogenous mixture of two or more substances
10
New cards
Components of a solution
solute and solvent
11
New cards
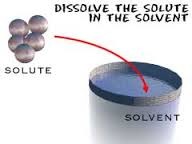
Solvents
The component of the solution that dissolved the other component in it. (Usually present in a larger amount.)
12
New cards
Solutes
The component of the solution that is dissolved in the solvent. (Usually present in the lesser quantity.)
13
New cards
examples of solutions
1. Sugar in water - solid in liquid solution.
2. Iodine in alcohol, 'tincture of iodine.' - liquid in liquid solution.
3. Aerated drinks - gas in liquid solutions.
4. Air - gas in gas solution.
2. Iodine in alcohol, 'tincture of iodine.' - liquid in liquid solution.
3. Aerated drinks - gas in liquid solutions.
4. Air - gas in gas solution.
14
New cards
Properties of solutions
1. The solution is a homogenous mixture.
2. Particles smaller than 1 nm, cannot be seen with the naked eye.
3. Do not scatter a beam of light due to its small particle size.
4. Solute particles cannot be separated from the mixture by filtration.
5. Solution is stable, particles do not settle down when left undisturbed.
2. Particles smaller than 1 nm, cannot be seen with the naked eye.
3. Do not scatter a beam of light due to its small particle size.
4. Solute particles cannot be separated from the mixture by filtration.
5. Solution is stable, particles do not settle down when left undisturbed.
15
New cards
Alloys
Mixtures of two or more metals or a metal and a non-metal that cannot be separated into their components by physical methods.
16
New cards
Why is an alloy considered a mixture?
It shows properties of its constituents and can have variable composition.
17
New cards
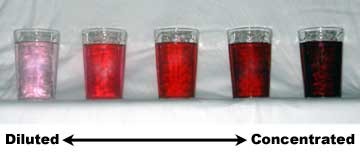
What are the type of solution based on the amount of solute present?
It can be called a dilute or concentrated or saturated solution.
18
New cards
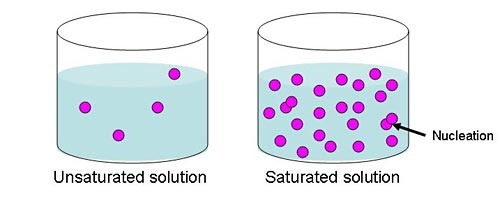
saturated solution
A solution that has dissolved as much solute as it is capable of dissolving at any particular temperature.
19
New cards
Solubility
The amount of solute present in the saturated solution at a given temperature.
20
New cards
unsaturated solution
The solution in which the amount of solute contained in the solution is less than the saturation level.
21
New cards
concentration of a solution
Amount of solute present in a given amount (mass or volume) of the solution.
22
New cards
Concentration of a solution =
Amount of solute / Amount of solution
23
New cards
Mass by mass concentration of a solution =
Mass of solute / mass of solution x 100%
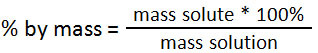
24
New cards
Mass by volume concentration of a solution =
Mass of solute / volume of solute x 100%
25
New cards
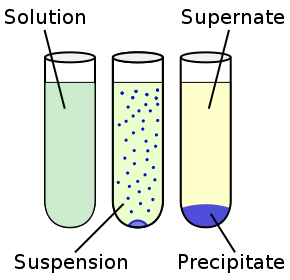
Suspensions
A heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
26
New cards
properties of suspensions
1. They are heterogeneous.
2. Particles are big, can be seen with the naked eye.
3. Particles scatter a beam of light through it and makes its path visible.
4. Unstable, can be separated with the process of filtration.
2. Particles are big, can be seen with the naked eye.
3. Particles scatter a beam of light through it and makes its path visible.
4. Unstable, can be separated with the process of filtration.
27
New cards
What happens when the particles in a suspension settle down?
The suspension breaks and it does not scatter light anymore.
28
New cards
Colloids
Heterogeneous mixtures that appear to be homogenous due to their small particle size.

29
New cards
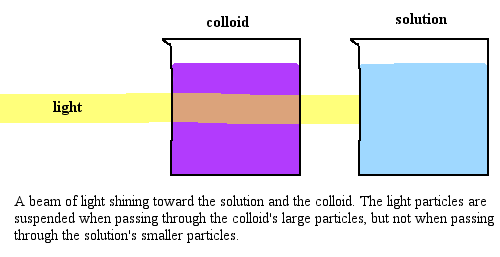
Tyndall effect
The scattering of a beam of light by colloidal particles.
30
New cards
Examples of Tyndall effect
1. When sunlight passes through the canopy of a dense forest,
Mist acts as colloid dispersed in air.
2. When we see dust particles in a small beam of light,
Dust and particles act as colloid dispersed in ai.
Mist acts as colloid dispersed in air.
2. When we see dust particles in a small beam of light,
Dust and particles act as colloid dispersed in ai.

31
New cards
properties of colloids
1. Heterogenous.
2. Particle size- bigger than that of a solution, smaller than that of a suspension and therefore cannot be seen with the naked eye.
3. Scatter a beam of light passing through it and make its path visible.
4. Do not settle down when left undisturbed, quite stable.
2. Particle size- bigger than that of a solution, smaller than that of a suspension and therefore cannot be seen with the naked eye.
3. Scatter a beam of light passing through it and make its path visible.
4. Do not settle down when left undisturbed, quite stable.
32
New cards
components of a colloid
1. Dispersed phase (solute).
2. Dispersion medium (solvent).
2. Dispersion medium (solvent).
33
New cards
dispersed phase
Solute-like component/dispersed particles in a colloid.
34
New cards
dispersion medium
Component in which dispersed phase is suspended in.
35
New cards
Colloids are classified according to
State of dispersing medium and dispersed phase
36
New cards
different types of colloids
1. Aerosol.
2. Foam.
3. Emulsion.
4. Sol.
5. Gel.
6. Solid Sol.
2. Foam.
3. Emulsion.
4. Sol.
5. Gel.
6. Solid Sol.
37
New cards
Aerosol
A type of colloid in which liquid drops or solid particles are spread throughout a gas.

38
New cards
Foam
A colloidal dispersion of a gas in a liquid.

39
New cards
Emulsion
A colloidal dispersion of a liquid in a liquid.

40
New cards
Sol
A colloidal dispersion of a solid in a liquid.
41
New cards
Gel
A colloidal dispersion of a liquid in a solid.

42
New cards
Solid sol
A solid dispersed within a solid. (certain metal alloys).
43
New cards
Why is separation necessary?
Separation makes it possible to study and use the individual components of a mixture.
44
New cards
How can heterogenous mixtures be separated?
Physical methods like handpicking, sieving, filtration.
45
New cards
How can we obtain coloured component (dye) from blue/black ink?
By Evaporation.
46
New cards
How do we separate volatile components from non-volatile ones?
By Evaporation.
47
New cards
What is a volatile component?
Something that can evaporate on heating.
48
New cards
How can we separate cream from milk?
By Centrifugation.
49
New cards
Principle of Centrifugation
When spun rapidly the denser particles are forced to the bottom and the lighter particles stay at the top.
50
New cards
applications of centrifugation
1. Used in diagnostic laboratories for blood and urine tests.
2. Used in fairies and homes to separate butter from cream.
3. Used in washing machines to squeeze out water from wet clothes.
2. Used in fairies and homes to separate butter from cream.
3. Used in washing machines to squeeze out water from wet clothes.
51
New cards
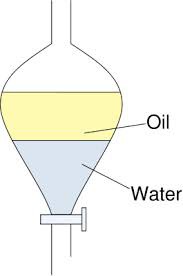
How can we separate a mixture of two immiscible liquids?
by using a separating funnel.
52
New cards
Applications of a separating funnel
1. To separate oil and water.
2. Iron from its ore.
2. Iron from its ore.
53
New cards
The principle of a separating funnel
Immiscible liquids separate out in layers, depending on their densities.
54
New cards
How can we separate mixtures that contain a sublimate volatile component and a non sublimate impurity?
By sublimation.
55
New cards
Solids that sublime
Ammonium chloride,
Camphor,
Naphthalene,
Anthracene.
Camphor,
Naphthalene,
Anthracene.
56
New cards
How do we separate dyes in black ink?
By using chromatography.
57
New cards
Chromatography
The separation of a mixture by passing it in solution through a medium in which the components move at different rates.
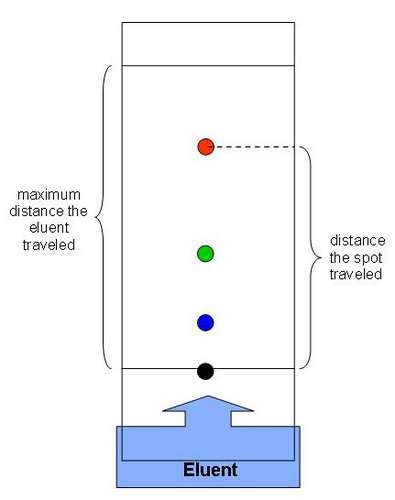
58
New cards
How do we separate solutes that dissolve in the same solvent?
by chromatography.
59
New cards
applications of chromatography
To separate
1. Colours in a die,
2. Pigments from natural colours,
3. Drugs from blood.
1. Colours in a die,
2. Pigments from natural colours,
3. Drugs from blood.
60
New cards
How can we separate a mixture of two miscible liquids?
By distillation
61
New cards
Distillation
The process of separating the components or substances from a liquid mixture by selective boiling and condensation.
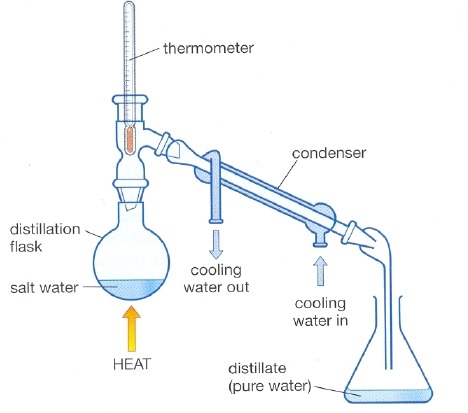
62
New cards
When is distillation used?
It is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
63
New cards
When is fractional distillation used?
To separate a mixture of two or more miscible liquids for which the difference in their boiling points is less than 25K.
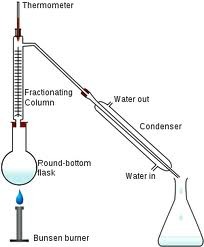
64
New cards
Differentiate between the apparatus used in fractional distillation and simple distillation.
Fractionating column is fitted in between the distillation flask and the condenser.
65
New cards
A simple fractionating column
Tube with glass beads,
glass beads provide a surface for the vapours to cool and condense repeatedly
glass beads provide a surface for the vapours to cool and condense repeatedly
66
New cards
How can we obtain different gases from air?
By distillation.
67
New cards
How can we obtain pure copper sulphate from an impure sample? How do we purify solids?
By Crystallization.
68
New cards
Crystallization
Process that separates a pure solid in the form of its crystals from a solution.

69
New cards
Why is crystallization preferred over evaporation?
1. Some solids decompose or some may get charred on heating to dryness.
2. Some impurities may remain dissolved even after evaporation.
2. Some impurities may remain dissolved even after evaporation.
70
New cards
application of crystallization
1. Purification of salt that we get from sea water.
2. Separation of crystals of alum from impure samples.
2. Separation of crystals of alum from impure samples.
71
New cards
physical change
a change of matter from one form to another without a change in composition. There is no change in the chemical nature of the substance.

72
New cards
chemical change
A change that occurs when one or more substances change into entirely new substances with different chemical properties. Also known as a chemical reaction.

73
New cards
Who was the first scientist to use the term element?
Robert Boyle in 1661
74
New cards
Who was the first to establish an experimentally useful definition of an element? What was it?
Antoine Laurent Lavoisier (1743-94)
Element- the basic form of matter that cannot be broken down into simpler substances by chemical reactions.
Element- the basic form of matter that cannot be broken down into simpler substances by chemical reactions.
75
New cards
Elements can be divided into
Metals, non-metals, metalloid
76
New cards
Metals
Substance composed of one or more metallic elements that is
shiny, hard, and malleable if solid.
shiny, hard, and malleable if solid.

77
New cards
Non-metals
Elements that are usually dull in appearance, poor conductors of heat and electricity.

78
New cards
Metalloids
Elements that have intermediate properties between those of metals and non-metals.
example - silicon.
example - silicon.

79
New cards
Elements that are liquid at room temperature are ------------.
mercury and bromine

80
New cards
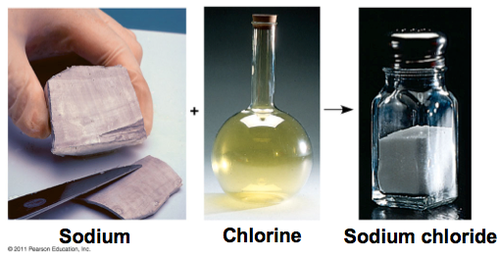
Compounds
A substance composed of two or more elects that chemically combined with one another in a fixed proportion.
example - water (H2O).
example - water (H2O).
81
New cards
Why is the sky blue?
The air molecules scatter blue light better than red light, so more blue light reaches our eyes.
82
New cards
Brownian motion
Zig-zag motion of colloidal particles is called Brownian motion.