ochem 24 metabolic pathways for lipids and amino acids
1/147
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
148 Terms
where is fat stored in the body
adipocytes
fat cells (adipocytes) store how much fat and in what form
unlimited and in the form of triacylglycerides
adipose tissue stored ___% of energy available in the body
85
where does digestion of fats begin
small intestines
role of bile salts in digestion of fats
emulsify the fat globules into smaller particles called micelles in the small intestines
pancreatic lipases role in digestion
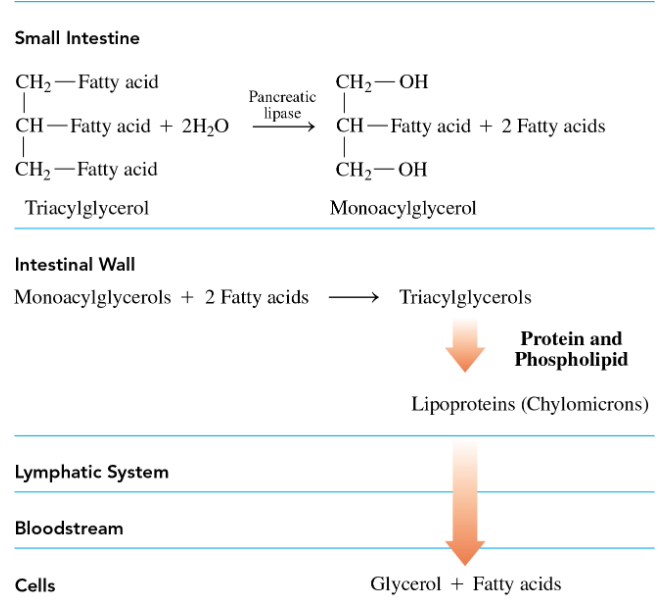
after bile salts emulsify the fat glubules into miccelles, pancreatic lypases hydrolyse ester bonds to form monoglycerols and free fatty acids which are small enough to be transported into the cells of the intestinal lining
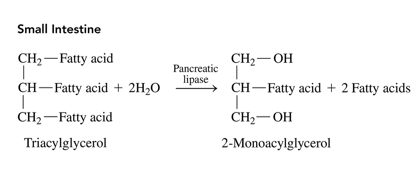
what occurs to the monoglycerols and free fatty acids in the intestinal lining
they recombine into tryacylglycerols
once triacylglycerols are in the intestinal lining, what occurs to them
phospholipids and proteins coat the fats, forming chylomicrons, which are transported to the cells of heart, muscle, and adipose tissues.
in the cells of the heart, muscle, and adipose tissue, what do lipases do
lipases hydrolyze triacylglycerols, forming glycerol and free fatty acids, which are oxidized to acetyl CoA molecules for ATP synthesis.
overall digestion of fats
in reference to fat, when blood glucose is depleted and glycogen stores are low
the process of fat utilization is stimulated.
when fat utilization is stimulated, what hormones are released and what do they do
•the hormones glucagon and epinephrine are secreted into the bloodstream, where they bind to receptors on the membrane of adipose tissue.
when glucagon and epinephrine are secreted into bloodstream, they activate what
hormone sensitive lipase
what does hormone-sensitive lipase do
within the fat cells catalyzes the hydrolysis of triacylglycerols to glycerol and free fatty acids
what happens to the glycerol and free fatty acids made from hydrolysis of the triacylglycerol
•diffuse into the bloodstream and bind with plasma proteins to be transported to the tissues, muscles, and fat cells.
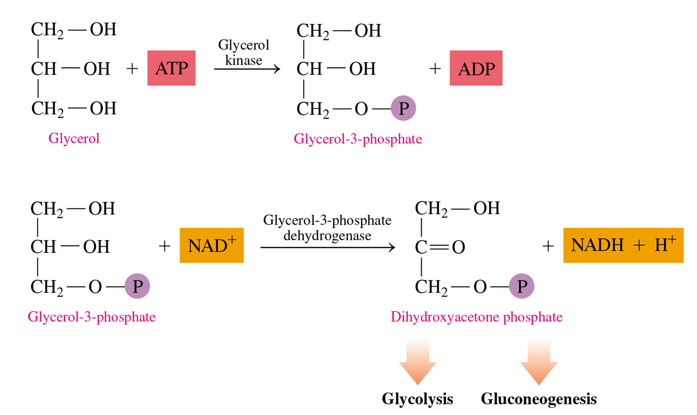
how to we metabolize glycerol
it is converted into dihydroxyacetone phosphate into 2 steps
uses ATP to give P to glycerol which turns it into glycerol-3-p
glycerol-3-p is oxidized at its -OH group into dihydroxyacetone phosphate (uses NAD+—> NADH)
this can then go into glycolysis or gluconeogenesis
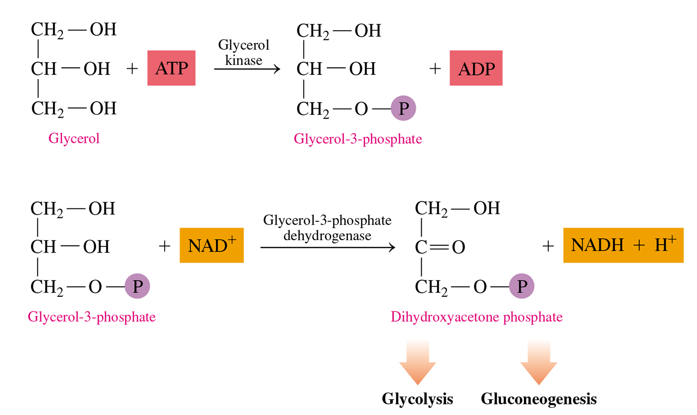
in the metabolism of glycerol how many ATP used and how many NADH are made
1 ATP in the first step to form glycerol-3-p
1 NADH in the second step to form dihydroxyacetone phosphate
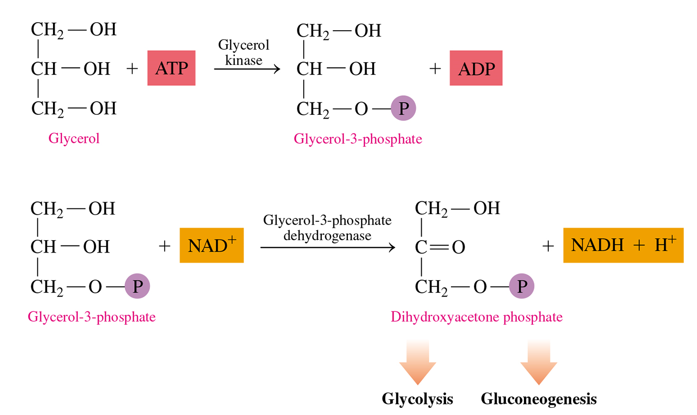
in the metabolism of glycerol, what do we end up creating
dihydroxyacetone phosphate, which is an intermediate in glycolysis and gluconeogenesis.
what is beta-oxidation
the catabolic reaction that breaks down a fatty acid by removing two-carbon segments containing the alpha and beta carbon from the carboxyl end of the fatty acid
what hormone activates beta oxidation
glucagon (wants energy in the bloodstream so wants us to break down fat molecules to make ATP)
a cycle of beta oxidation produces what (compounds)
acetyl CoA and a FA that is 2C’s shorter than it was
how many cycles does a fatty acid go through in beta oxidation
repeats until the original FA is completely degraded into 2-C units that form acetyl CoA to enter the citric acid cycle
where in the cell do we find fatty acids
cytosol
where does beta oxidation take place in
matrix of mitochindria
what must happen to the FA before beta oxidation can occur (not location)
it is combined with HS-CoA to yield high energy fatty acyl CoA (ACTIVATED)
what does the activation of a FA for beta oxidation use
- uses ATP to use the energy from the hydrolysis of it to AMP
- has HS-CoA added to FA
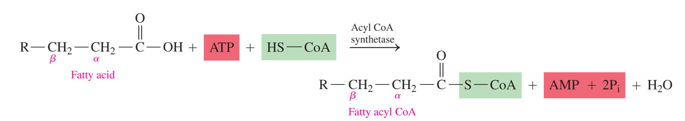
what is the activated form of a fatty acid
fatty acyl CoA (fatty acid with a CoA)
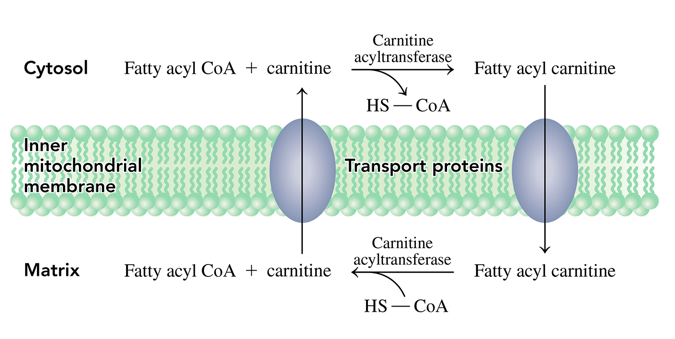
how does the fatty acid get from the cytosol to the mitochondrial matrix
it uses the carnitine shuttle where the fatty acyl group is transferred to the hydroxyl group of carnitine to produce fatty acyl carnitine which can pass through the inner mitochondrial membrane into the matrix
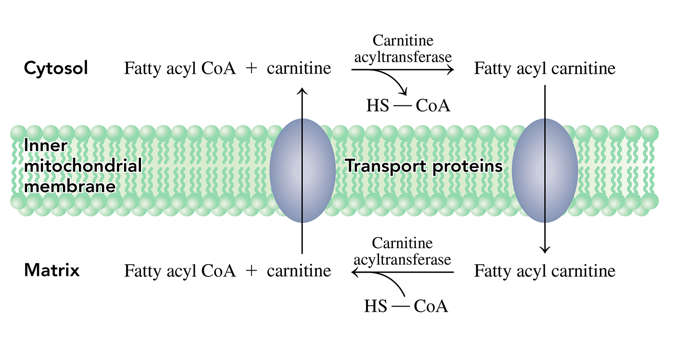
once in the mitochondrial matrix, how do we get fatty acyl CoA again
- then when in the mitochondria a reverse reaction occurs where the fatty acyl group is transferred to CoA to reform fatty acyl CoA
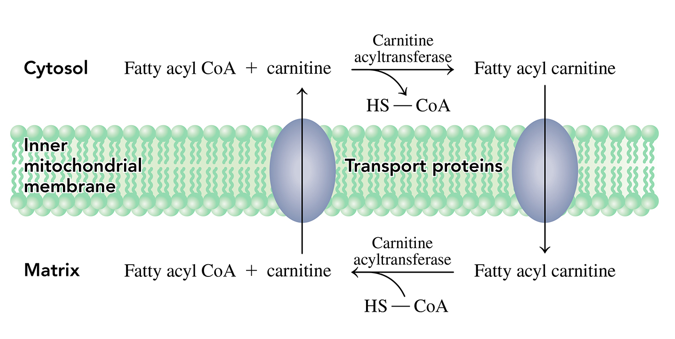
what is the carnitine shuttle
how a fatty acyl can get from the cytosol into the mitochondrial matrix.
- Fatty acyl CoA has its fatty acyl group is transferred to the hydroxyl group of carnitine to produce fatty acyl carnitine which can pass through the inner mitochondrial membrane into the matrix
- then when in the mitochondria a reverse reaction occurs where the fatty acyl group is transferred to CoA to reform fatty acyl CoA
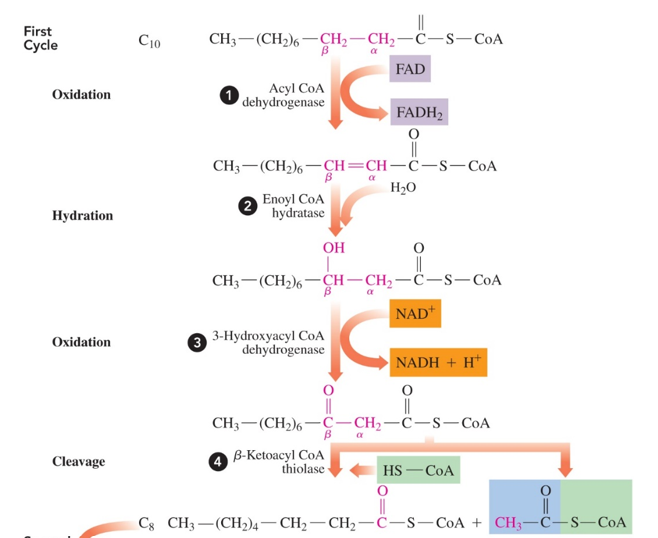
beta oxidation has how many reactions
4 reactions in each cycle
in each cycle of beta oxidation, what is produced
- 1 NADH
- 1 FADH2
- 1 fatty acid chain that is 2C’s shorter
why is it called beta oxidation?
because you end up breaking the bond between the beta and alpha carbon of the fatty acid chain, and these 2 carbons undergo a series of oxidation reactions that end up producing NADH and FADH2
when re usually refer to beta oxidation, we are referring to what kind of fatty acids
saturated fatty acids (no double bonds)
beta oxidation reactions
1.fatty acyl CoA is oxidized to form a trans C=C bond between the alpha and beta carbon, producing FADH2 (fatty acyl Coa—> trans-enoyl CoA)
2.trans-enoyl Coa is then hydrated to break the = bond and add an OH to the beta carbon (trans-enoyl CoA —> 3-hydroxyacyl CoA)
3.3-hydroxyacyl CoA is oxidized to have the OH on the beta carbon become a carbonyl (c=o) group, producing NADH (3-hydroxyacyl CoA—> beta-ketoacyl CoA)
4. beta-ketoacyl CoA then split between the beta and alpha carbon, forming a fatty acyl CoA that is 2 carbons shorter (and has CoA added to it) and an acetyl CoA
how do you know how many cycles a fatty acid goes through
•The number of carbons in a fatty acid determines the number of times the cycle repeats and the number of acetyl CoA units it produces.
The total number of times the cycle repeats is one fewer than the total number of acetyl groups it produces.
what about off numbered fatty acids
-go through the same 4 steps of beta oxidation
BUTTTTT
-in final cycle, the remaining fatty acyl CoA is cleaved to yield a propionyl CoA (C3) group and an acetyl CoA (so a 3 carbon molecule and a 2 C molecule)
what type of fatty acids does normal beta oxidation occur with
saturated (no = bonds)
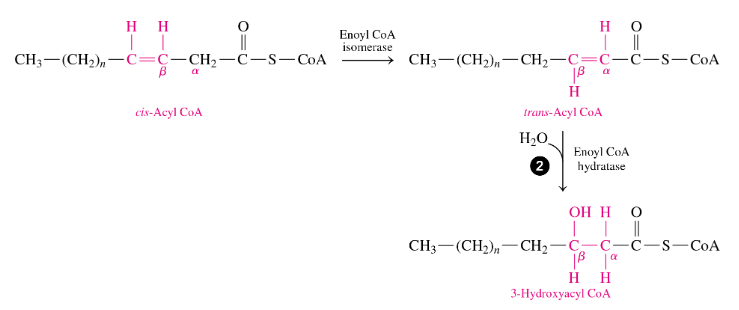
how does beta oxidation of unsaturated fatty acids differ from that of saturated
they have one or more cis double bonds so
•before starting oxidation, an isomerase converts a cis double bond to a trans double bond between the α and β carbons (moves the = bond to be between the α and β C’s and makes it trans) so the fatty acid can undergo hydration.
•It forms a product that enters β oxidation at reaction 2, so the energy released by the β oxidation of an unsaturated fatty acid is slightly less because no FADH2 is produced in that cycle—→ so no FADH made in every cycle that had a cis double bond)
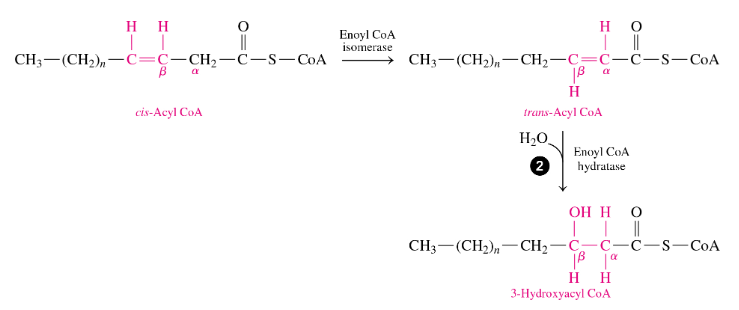
when we have a unsat fatty acid undergo beta-oxidation, what outcome is different
for every = bond, no FADH is made in that cycle it is involved in
-so if we have a 18:1, this undergoes 8 cycles and instead of 8FADH being made like in 18:0, only 7FADH are made)
how many acetyl coA are formed for a fatty acid
-if even number, ½ amount of carbons
-if odd numbered, ½ amount of C minus 1 (last cycle)
how many cycles does a fatty acid undergo in beta oxidation
1 less than the amount of fatty acids made; 1 less than ½ the amount of carbons
-if we have a 16C FA, it makes 8 acetyl Coa, so it goes through 7 cycles
activation of fatty acid uses how many ATPs
2
NADH makes how many ATP molecules
2.5
FADH makes how many ATP
1.5
acetyl CoA goes where
the citric acid cycle
1 cycle of the citric acid cycle makes how many ATPs
10 ATPs
so how do we calculate total ATP production for beta oxidation of a fatty acid
-2 ATP from activation
10x # of acetyl Coa made
2.5 x # NADH made
1.5 x #FADH2 made
then add all these and you get your total ATP
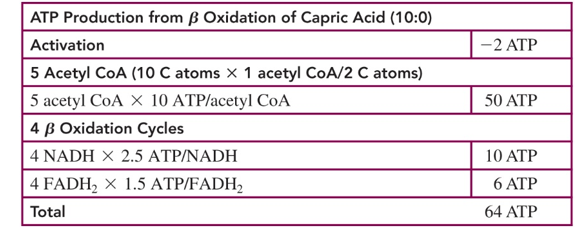
when we do not have carbohydrates available to break down for energy, what do we use instead
fatty acids
what do fatty acids break down to
acetyl coA
can acetyl coA be made into pyruvate
NO
so what do with the acetyl coA formed by the oxidation of fatty acids
combine them to form ketone bodies
why do ketone bodies form?
because when we have to much acetyl coA produced due to the oxidation of fatty acids, it acculates in the liver because the citric acid cycle cannot process ot fast enough, so they combine through ketogenesis to form ketone bodies
what is ketogenesis
the pathway where acetyl coA compounds combine to form ketone bodies
how many reactions are in ketogenesis
4 reactions
how ketogenesis begin
2 acetyl CoA’s combine to form acetoacetyl coA by losing 1 coA (condensation)
- reverse to last step of beta oxidation
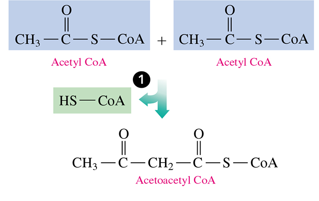
once we have acetoacetyl CoA,
we hydrolyze it by removing the CoA and form acetoacetate
acetoacetate can then become what
it can either become beta hydroxybutarate by being reduced (by NADH+) OR
it can be decarboxylated to yield acetone (and CO2)
-both of these possible products are ketone bodies
in ketogensis we begin with
2 acetyl CoA’s
in ketonegenesis we end with
a ketone body,
-either beta-hydroxybutyrate through reduction by NADH+
-or acetate by losing a carbon as CO2
what is ketosis
high accumulation of ketones (which are acidic) causes blood pH to be below 7.4
-occurs with diabetes, high fat diets, and starvation
what is diabetes (insulin, glucose, effect on fats)
when insulin does work properly and glucose levels are not enough for energy needs, so fats are used instead and broken down to acetyl COA in whcih the buildup activates ketogensis to produce ketone bodies
type 1 vs type 2 vs gestational
-type 1 is usually seen start in children and is when pancreas does not make enough insulin due to mutation or viral infection
-type 2 is usually seen in adults and is when insulin is made but the insulin receptors are not responsive
-gestational occurs during pregnancy but blood glucose levels go back to normal after baby is born
in diabetes, insufficient amounts glucose causes liver cells to
make glucose through gluconeogenesis which increases levels of acetyl coA —> ketogenesis—> ketosis
when we have consumed enough energy and our glycogen stores are full, the acetyl coA from breakdown of carbs and FAs is used
to make FAs in the cytosol
fatty acid synthesis is also called
lipogenesis
lipogenesis primarily makes what fatty acid
16 carbon, saturated FA: palmitic acid
how does fatty acid synthesis work (big picture)
linking acetyl groups to form a 16-C FA (palmitic acid) by adding 2-carbon acetyl units to growing chain
is the synthesis of fatty acids the reverse of beta oxidation
NOOOOOOO
- different location (cytosol vs mitochondrial matrix)
-different coenzymes (NAPDH vs FAD and NAD+)
-different enzymes
what do we link together in fatty acid synthesis (the goal of each cycle)
add a acyl group to the growing acyl chain
what FA do we usually produce through this
palmitate (16 C’s)
what must occur before FA synthesis can begin
the acyl compounds that will be added MUST BE ACTIVATED into activated carriers
how is an acyl compound activated
an acyl carrier protein (HS- ACP) binds to it
acyl carrier protein
HS- ACP
-similar to CoA (aminoethanethiol, pantothenic acid, and phosphate) but has a protein attached to the protein
-binds to the acyl compound to make it into an active carrier
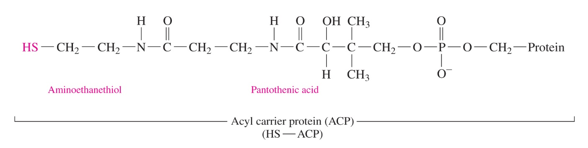
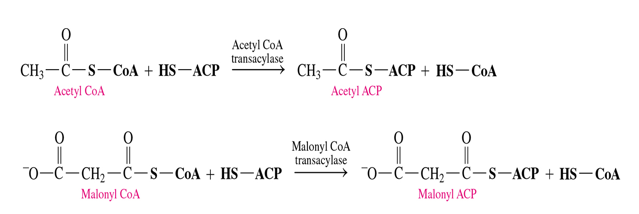
what 2 compounds do we need to activate with ACP?
acetyl CoA and malonyl CoA
malonyl CoA is not already synthesized in the body so how do we make it
acetyl coa + bicarbonate —> malonyl CoA
- uses 1 ATP
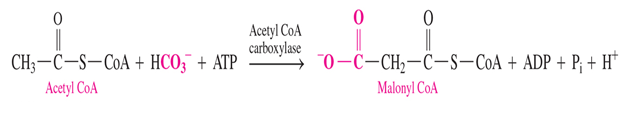
what do malonyl CoA and Acetyl CoA turn into when activated
malonyl CoA—> malonyl ACP
acetyl CoA—> acetyl ACP
how many reactions are in synthesis of FA’s
4 reactions
where does synthesis of FA’s occur
cytosol (so anaerobic)
in FA synthesis what do you begin with (what compound)
acetyl CoA (which is then turned into acetyl ACP and combined with malonyl CoA)
what is used for fatty acid synthesis
NADPH
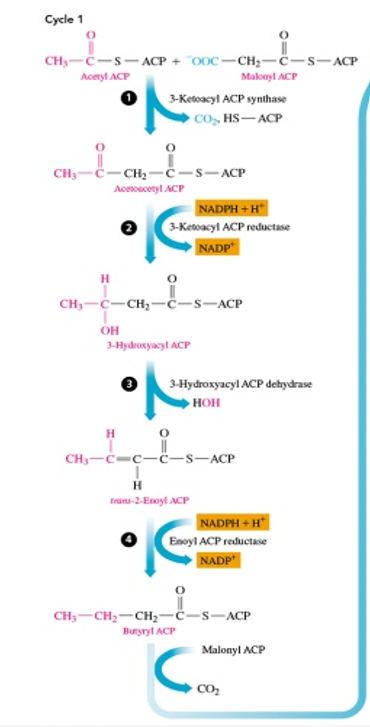
lipogenesis steps
-acetyl ACP and malonyl ACP are combined to form acetoacetyl ACP by malonyl ACP losing a carbon and acetyl CoA losing ACP, which releasing CO2 and a ACP
-acetoacetyl ACP is reduced to 3-hydroxyacyl ACP, making NADP+
-3-hydroxyacyl ACP is then dehydrated (loses H2O) to make trans-2-enoyl ACP (so loses water and forms a = bond between those carbons)
-trans-2-enoyl ACP is reduced into butyryl ACP (no c=c bond) which is 2 C’s longer, and NADP+ is made
-then cycle repeats by adding in a malonyl ACP and then repeating the steps to make if longer
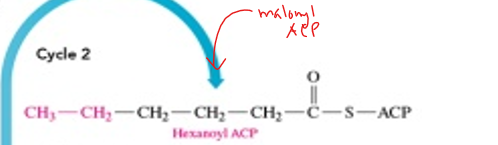
how many cycles occur in fatty acid synthesis
-most of the time the FA made is palmitate which has 16 C’s, so the cycle repeats 7 times
-if we need to make shorter chains it will just stop earlier
-if need longer it will continue but with with special enzymes to add the C’s as this process is only for up to 16 Cs
in cycle 2, what do we end up making
hexanoyl ACP
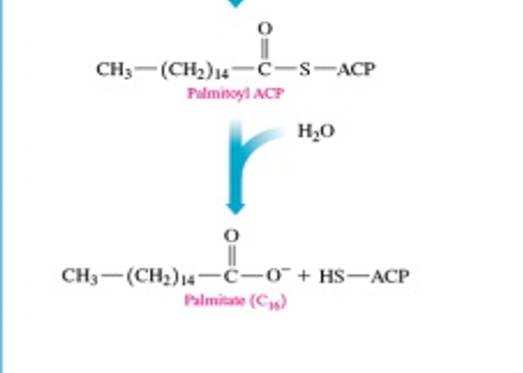
at the end of lipogenesis, what do we do with palminoyl ACP
hydrolyse the ACP off to make palmitate
what are the 4 reactions we see
1. condensation
2. reduction
3. dehydration
4. reduction
in making malonyl Coa (needed to make malonyl ACP) what do we need
1 ATP used
for each cycle of fatty acid synthesis, what do we make/ use
- use 2 NADPH
-make FA that is 2C longer
in the fatty acid synthesis of palmitate, what do end up producing/ using
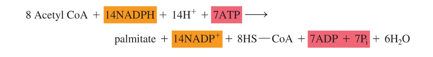
-use 8 acetyl CoA, use 14 NADPH, and 7 ATP
-make palmitate, 14 NADH+, 7 ADPs (and release 8 CoA)
if we need to make shorter FA chains (< 16C’s)
•shorter fatty acids are released earlier in the fatty acid synthesis process before there are 16 carbon atoms in
the chain.
how is FA synthesis regulated?
by insulin
if we need to make longer FA chains (>16C’s)
•longer fatty acids are produced with special enzymes that add two-carbon acetyl units to the carboxyl end of the fatty acid chain.
how does insulin regulate fatty acid synthesis
-when blood glucose is high, it is released and moves the glucose into the cells to stimulate glycolysis and oxidation of pyruvate (into acetyl CoA)
-the buildup of acetyl CoA initiates fatty acid synthesis (making fat)
in these circumstances, what happens to the transport of acyl group into the matrix of mitochondria
they are blocked to prevent their oxidation (which leads to energy production) so they can undergo fatty acid synthesis in adipose tissue
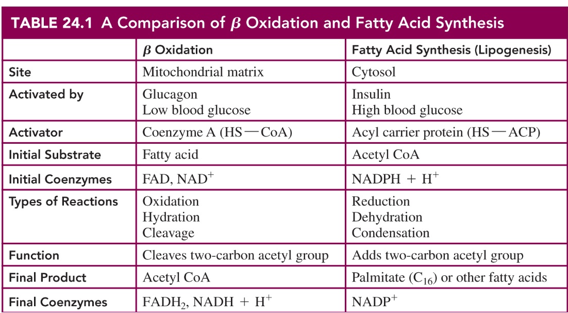
beta oxidation vs Fatty acid synthesis
NOW WE START PROTEINS
when carbs and lipids are not available,
amino acids are degraded to substrates that enter energy-producing pathways
where does protein digestion begin
in the stomach
HCL in the stomach (the acidity) does what
denatures the proteins
pepsin
hydrolyses the proteins into polypeptide chains