CH07 Reactions in Aqueous Sol
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Reactions in Aqueous Solutions
• Chemical reactions that are most important to us occur in
water—in aqueous solutions,
➡ In this chapter we will study some common types of
reactions that take place in water,
➡ Driving forces that make these reactions occur,
➡ Learn how to predict the products for these reactions, and
➡ How to write various equations.
Reactions in Aqueous Solutions
Four Driving Forces Favor Chemical Change
1. Formation of a solid
2. Formation of water
3. Transfer of electrons
4. Formation of a gas
Reactions in Aqueous Solutions
Precipitation Reactions
Acid–Base Reactions
Oxidation–Reduction Reactions
Precipitation
The process of formation of a solid during a
chemical reaction
– The solid formed during this reaction is called a precipitate
– The reaction is known as a precipitation reaction
What Happens When an Ionic Compound Dissolves in Water?
• The ions separate and move around independently
➡ Strong electrolyte: Substance whose each unit produces separated ions when dissolved in water
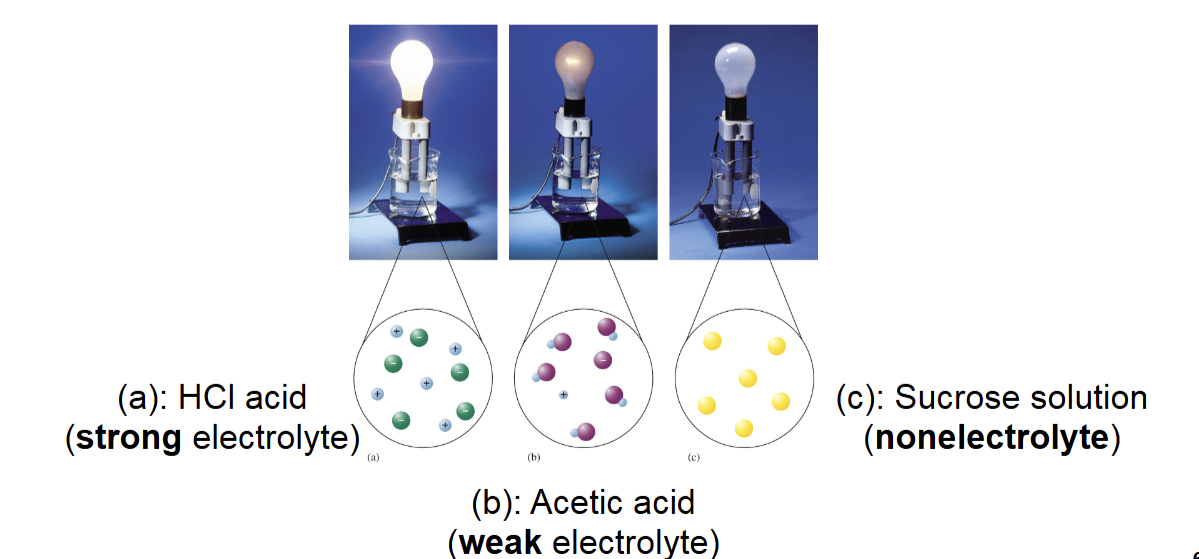
What Happens When an Ionic Compound Dissolves in Water?
• Electrolyte is a substance that dissolves in water to yield a
solution that conducts electricity.
• By contrast, a nonelectrolyte is a substance that dissolves in
water to yield a solution that does not conduct electricity.
• An electrolyte that dissociates completely is known as a
strong electrolyte.
• A weak electrolyte is a compound that produces ions upon
dissolving but exists in solution predominantly as molecules
that are not ionized.
Precipitation Reactions
▪ Soluble – solid dissolves in solution; (aq) is used in reaction
equation.
▪ Insoluble – solid does not dissolve in solution; (s) is used in
reaction equation.
➡ Insoluble and slightly soluble are often used
interchangeably.
Formula Equation (Molecular Equation)
▪ Gives the overall reaction stoichiometry but not necessarily
the actual forms of the reactants and products in solution.
▪ Reactants and products generally shown as compounds.
▪ Use solubility rules to determine which compounds are
aqueous and which compounds are solids.
Complete Ionic Equation
• All substances that are strong electrolytes are represented as ions
➡ Spectator ions: Ions that do not participate directly in a reaction
in solution
Net Ionic Equation
An equation that includes only those components that are
directly involved in the reaction
• Note: Spectator ions are not included in the net ionic equation
Summarizing Aqueous Equations
• Molecular equation is a chemical equation showing the
complete, neutral formulas for every compound in a reaction.
• Complete ionic equation is a chemical equation showing all
of the species as they are actually present in solution: strong
electrolytes are therefore represented as their component ions.
• Net ionic equation is an equation showing only the species
that actually change during the reaction.
Arrhenius Acids and Bases
Strong acid: A strong electrolyte that produces H+ ions
(protons) when it is dissolved in water
Strong base: A substance that produces hydroxide ions (OH−)
in water
– Most common examples: NaOH and KOH
• The products of the reaction of a strong acid and a strong
base are water and a salt
– Salt ⇒ Ionic compound
• Net ionic equation
– H +(aq) + OH −(aq) → H 2 O(l)
'
• Reaction of H+ and OH − is called an acid–base reaction
– H + ⇒ acidic ion
– OH − ⇒ basic ion
Summary of Strong Acids and Strong Bases
1. Common strong acids include aqueous solutions of HCl,
HNO3 , and H 2 SO4
2. A strong acid is a substance that completely dissociates
(ionizes) in water (into an H+ ion and an anion)
3. A strong base is a metal hydroxide compound that is very
soluble in water
– The most common strong bases are NaOH and KOH, which
completely dissociate into separated ions (Na+ and OH – or K + and
OH – ) when they are dissolved in water
Summary of Strong Acids and Strong Bases
4. The net ionic equation for the reaction of a strong acid and a
strong base is always the same:
➡ it shows the production of water
5. In the reaction of a strong acid and a strong base, one
product is always water and the other is always an ionic
compound called a salt, which remains dissolved in the water
– This salt can be obtained as a solid by evaporating the water
6. The reaction of H + and OH – is often called an acid–base reaction
(or Neutralization Reaction), where H+ is the acidic ion and OH– is
the basic ion
Oxidation–Reduction Reaction
• A reaction that involves a transfer of electrons
➡ 2Mg(s) + O2 (g) → 2MgO(s)
• Reactions between metals and nonmetals involve a transfer
of electrons from the metal to the nonmetal
• In this process, zinc atoms are oxidized (they lose electrons)
and copper ions are reduced (they gain electrons).
Characteristics of Oxidation–Reduction Reactions
1. A metal–nonmetal reaction can always be assumed to be an
oxidation–reduction reaction, which involves electron transfer
2. Two nonmetals can also undergo an oxidation–reduction
reaction
– At this point, we can recognize these cases only by looking for O2
as a reactant or product
– When two nonmetals react, the compound formed is not ionic
Driving Forces for a Reaction
• Formation of a solid
• Formation of water
• Transfer of electrons
• Formation of a gas
Precipitation Reaction
• Formation of a solid when two solutions are mixed
• Double-displacement reaction
✴ AB + CD → AD + CB
Acid–Base Reaction
• Involves an H+ ion that ends up in the product as water
Combustion Reactions
• Involve oxygen and produce energy (heat) so rapidly that a
flame results
➡ None of the reactants or products is ionic
➡ Special class of oxidation–reduction reactions
Synthesis (Combination) Reactions
One of the most important activities in chemistry is the
synthesis of new compounds.
• Involve the formation of a compound from simpler materials
– Special class of oxidation–reduction reactions
Decomposition Reactions
• Occur when a compound is broken down into simpler
substances
• This is usually accomplished by heating or by the application of
an electric current.
– Special class of oxidation–reduction reactions