Chirality II
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
assigning r and s enantiomers
absolute configuration defines the exact spatial arrangement of the atoms in the chiral molecule
Cahn-Ingold-Prelog rules
locate the chiral carbon and assign highest(1) to lowest (2) priority to the 4 atoms bounded to the chiral carbon according to their atomic numbers.
if their are 2 or more identical atoms. then priorities of the substituents are the established by the stes of atoms one further bond away.
for multiple bonds the order is triple>double>single
assigning configuration to a 3D chiral molecule
rotate molecule so that the lowest priority group is at the back. draw arrow from highest to lowest priority. R enantiomer is clockwise. S enantiomer is anti-clockwise
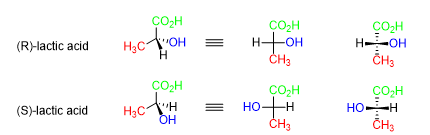
Fischer projections
tetrahedral carbon represented by two crossed lines
horizontal line is coming out of the plane of the page (towards you)
vertical line is going back behind the plane of the paper (away from you)
can only rotate by 180 degrees