PREPARATIONS
1/197
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
198 Terms
Non-Medicated Aromatic Water
category of cinnamon water
synonym of cinnamon water
aqua cinnamon
synonym of cinnamon water
saigon cassia
Cinnamon Water
It is a clear, colorless, saturated aqueous solution with characteristic odor and taste of cinnamon.
flavoring vehicle
use/s of cinnamon water
cinnamon oil and purified water (qs ad)
formulation of cinnamon water
cinnamon oil
Procedure for Cinnamon Water:
Add ___________ to water
Shake vigorously for ____________ in a bigger bottle
Let the mixture stand for _________________
______ using a filter paper that was wetted with water
Stop filtering when a _____________ is obtained
Add q.s water through ___________
1 = ?
15 minutes
Procedure for Cinnamon Water:
Add ___________ to water
Shake vigorously for ____________ in a bigger bottle
Let the mixture stand for _________________
______ using a filter paper that was wetted with water
Stop filtering when a _____________ is obtained
Add q.s water through ___________
2 = ?
12 hours or longer
Procedure for Cinnamon Water:
Add ___________ to water
Shake vigorously for ____________ in a bigger bottle
Let the mixture stand for _________________
______ using a filter paper that was wetted with water
Stop filtering when a _____________ is obtained
Add q.s water through ___________
3 = ?
Filter
Procedure for Cinnamon Water:
Add ___________ to water
Shake vigorously for ____________ in a bigger bottle
Let the mixture stand for _________________
______ using a filter paper that was wetted with water
Stop filtering when a _____________ is obtained
Add q.s water through ___________
4 = ?
clear liquid
Procedure for Cinnamon Water:
Add ___________ to water
Shake vigorously for ____________ in a bigger bottle
Let the mixture stand for _________________
______ using a filter paper that was wetted with water
Stop filtering when a _____________ is obtained
Add q.s water through ___________
5 = ?
filter paper
Procedure for Cinnamon Water:
Add ___________ to water
Shake vigorously for ____________ in a bigger bottle
Let the mixture stand for _________________
______ using a filter paper that was wetted with water
Stop filtering when a _____________ is obtained
Add q.s water through ___________
6 = ?
white
cinnamon water label
30 mL amber bottle
cinnamon water container
Direct Method
method of preparation used in cinnamon water
tight and light-resistant container
Cinnamon water should be stored in a _________________________ to protect it from light and excessive volatilization.
Medicated Aromatic Water
category of concentrated peppermint water
synonym of concentrated peppermint water
aqua menthos piperitas
synonym of concentrated peppermint water
Brandy Water
Concentrated Peppermint Water
A clear, saturated solution of peppermint oil in purified water. A colorless aqueous solution with a characteristic odor taste of peppermint.
carminative and flavorant
use/s of concentrated peppermint water
Peppermint Oil, 90% ethyl alcohol, purified talc, purified water, qs ad
formulation of concentrated peppermint water
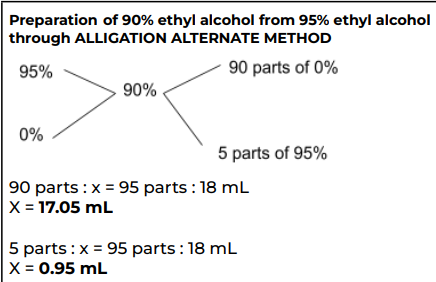
Prepare 18 mL of 90% ethyl alcohol from 95% ethyl alcohol through allegation alternate
90% Ethanol
Procedure for Concentrated Peppermint Water:
Dissolve peppermint oil in _______________
Add purified ______ that has been triturated
Add around _______ of water
Agitate for _________
__________ using a filter paper wetted with water
Add ________ to the desired volume.
1 = ?
talc
Procedure for Concentrated Peppermint Water:
Dissolve peppermint oil in _______________
Add purified ______ that has been triturated
Add around _______ of water
Agitate for _________
__________ using a filter paper wetted with water
Add ________ to the desired volume.
2 = ?
20mL
Procedure for Concentrated Peppermint Water:
Dissolve peppermint oil in _______________
Add purified ______ that has been triturated
Add around _______ of water
Agitate for _________
__________ using a filter paper wetted with water
Add ________ to the desired volume.
3 = ?
10 minutes
Procedure for Concentrated Peppermint Water:
Dissolve peppermint oil in _______________
Add purified ______ that has been triturated
Add around _______ of water
Agitate for _________
__________ using a filter paper wetted with water
Add ________ to the desired volume.
4 = ?
Filter
Procedure for Concentrated Peppermint Water:
Dissolve peppermint oil in _______________
Add purified ______ that has been triturated
Add around _______ of water
Agitate for _________
__________ using a filter paper wetted with water
Add ________ to the desired volume.
5 = ?
water
Procedure for Concentrated Peppermint Water:
Dissolve peppermint oil in _______________
Add purified ______ that has been triturated
Add around _______ of water
Agitate for _________
__________ using a filter paper wetted with water
Add ________ to the desired volume.
6 = ?
white
red or white label? concentrated peppermint water
30 mL amber bottle
container for concentrated peppermint water
alternate solution method
method of preparation for concentrated peppermint water
90% ethyl alcohol
solvent used in concentrated peppermint water
talc
used as a dispersing agent in concentrated peppermint water
rapid saturation
Talc ensures:
a. More _______________ of water
b. Forms an ______________ thus producing a clear solution
a = ?
efficient filter bed
Talc ensures:
a. More _______________ of water
b. Forms an ______________ thus producing a clear solution
b = ?
topical solution
category of calcium hydroxide topical solution
synonym of calcium hydroxide topical solution
lime water
Calcium Hydroxide Topical Solution
Clear, colorless solution with an alkaline taste
solution is generally employed with other ingredients in dermatological solution & lotion
astringent
used as protector in lotion preparation and as an emulsifying agent
use/s of calcium hydroxide topical solution
calcium hydroxide, purified water qs ad
formulation of calcium hydroxide topical solution
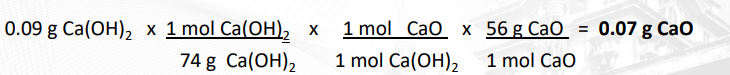
Calculation of amount of CaO (lime) from Ca(OH)2:
CaO + + H2O → Ca(OH)2
Amount? 0.09 g
MW: 56 g/n 74 g/n
reacting ratio: 1 mol CaO = 1mol Ca(OH)2
Slake
Procedure for Calcium Hydroxide Topical Solution:
__________ CaO (addition of water with the liberation of heat to form a crumbly material in a mortar/ beaker ) covered with watch glass
Let it stand for _______________
Add ___________ & agitate for 1 hour to dissolve the CaO totally
Filter, collect only the ___________________
Add sufficient amount of cold water up to the _______________ (no bubbles inside)
A ______________ solution should be achieved
1 = ?
5 minutes
Procedure for Calcium Hydroxide Topical Solution:
__________ CaO (addition of water with the liberation of heat to form a crumbly material in a mortar/ beaker ) covered with watch glass
Let it stand for _______________
Add ___________ & agitate for 1 hour to dissolve the CaO totally
Filter, collect only the ___________________
Add sufficient amount of cold water up to the _______________ (no bubbles inside)
A ______________ solution should be achieved
2 = ?
cold water
Procedure for Calcium Hydroxide Topical Solution:
__________ CaO (addition of water with the liberation of heat to form a crumbly material in a mortar/ beaker ) covered with watch glass
Let it stand for _______________
Add ___________ & agitate for 1 hour to dissolve the CaO totally
Filter, collect only the ___________________
Add sufficient amount of cold water up to the _______________ (no bubbles inside)
A ______________ solution should be achieved
3 = ?
supernatant liquid
Procedure for Calcium Hydroxide Topical Solution:
__________ CaO (addition of water with the liberation of heat to form a crumbly material in a mortar/ beaker ) covered with watch glass
Let it stand for _______________
Add ___________ & agitate for 1 hour to dissolve the CaO totally
Filter, collect only the ___________________
Add sufficient amount of cold water up to the _______________ (no bubbles inside)
A ______________ solution should be achieved
4 = ?
brim of the container
Procedure for Calcium Hydroxide Topical Solution:
__________ CaO (addition of water with the liberation of heat to form a crumbly material in a mortar/ beaker ) covered with watch glass
Let it stand for _______________
Add ___________ & agitate for 1 hour to dissolve the CaO totally
Filter, collect only the ___________________
Add sufficient amount of cold water up to the _______________ (no bubbles inside)
A ______________ solution should be achieved
5 = ?
clear, colorless
Procedure for Calcium Hydroxide Topical Solution:
__________ CaO (addition of water with the liberation of heat to form a crumbly material in a mortar/ beaker ) covered with watch glass
Let it stand for _______________
Add ___________ & agitate for 1 hour to dissolve the CaO totally
Filter, collect only the ___________________
Add sufficient amount of cold water up to the _______________ (no bubbles inside)
A ______________ solution should be achieved
6 = ?
red
Red or White Label? Calcium Hydroxide Topical Solution
30 mL flint bottle
Storage: Calcium Hydroxide Topical Solution
simple solution method
method of preparation for calcium hydroxide topical solution
well-filled, tightly stoppered containers to deter the absorption of CO2
The calcium hydroxide topical solution should be stored in ______________________________________________ and should be kept in a cool place to maintain an adequate concentration of dissolved solute.
Iodine
very reactive material that stains jewelries so in weighing, a porcelain spatula and watch glass must be used
1:2950
solubility of iodine in water
Potassium Iodide
solubilizing agent
converts I2 to I3 (triiodocomplex) which makes it soluble in water
topical solution
category of strong iodine solution
synonym of strong iodine solution
Lugol’s Solution
synonym of strong iodine solution
Aqueous Iodine Solution
synonym of strong iodine solution
Solution of Iodine
synonym of strong iodine solution
Liquor iodi
Strong Iodine Solution
A liquid with a deep brown color and odor of iodine
strong iodine solution
Use/s:
treatment of thyrotoxicosis
antigoitrogenic
germicidal
fungicidal
antiseptic keratolytic
iodine, potassium iodide, purified water qs ad
formulation of strong iodine solution
potassium iodide
Procedure of Strong Iodine Solution:
Dissolve _______________ in 3mL of water
Add __________ to the mixture
Add sufficient amount of ______
1 = ?
iodine
Procedure of Strong Iodine Solution:
Dissolve _______________ in 3mL of water
Add __________ to the mixture
Add sufficient amount of ______
2 = ?
water
Procedure of Strong Iodine Solution:
Dissolve _______________ in 3mL of water
Add __________ to the mixture
Add sufficient amount of ______
3 = ?
red label
Red or White Label? Strong Iodine Solution
30 mL amber bottle
container for strong iodine solution
Simple Solution
method of preparation of strong iodine solution
porcelain spatula
In weighing the iodine crystals, use ______________ instead of stainless because it reacts with metal.
Magnesium
naturally occurring mineral that is important for many systems in the body especially the muscles and nerves
saline cathartic
category of magnesium citrate oral solution
synonym of magnesium citrate oral solution
citrate
synonym of magnesium citrate oral solution
citrate of magnesia
synonym of magnesium citrate oral solution
magnessi citralis
synonym of magnesium citrate oral solution
lemonada purgante
Magnesium Citrate Oral Solution
It is a colorless to slightly yellow, clear effervescent liquid having a sweet acidulous taste and a lemon flavor
Saline Cathartic
use of magnesium citrate oral solution
increases water in the intestines
how does magnesium citrate oral solution induce defecation?
Magnesium Citrate, Citric Acid, Syrup, Talc, Lemon Oil, Potassium Bicarbonate, Purified water qs ad
formulation of magnesium citrate oral solution
marking
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
1 = ?
citric acid
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
2 = ?
MgCo3.Mg(OH)2.5H20
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
3 = ?
citric acid
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
4 = ?
syrup
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
5 = ?
lemon oil with talc
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
6 = ?
6 to 5
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
7 = ?
sterilized and calibrated bottle
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
8 = ?
water
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
9 = ?
cotton
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
10 = ?
KHCO3
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
11 = ?
cork
Procedure for Magnesium Citrate Oral Solution:
1. Calibrate the bottle first by ________ the 50mL level
2. In container 1, dissolve __________ in 21mL of water
3. In container 2, mix _________________ in 14mL of water
4. Mix magnesium carbonate hydroxide pentahydrate solution with _______. Stir until it is totally dissolved.
5. Add _________ as a sweetening agent and heat mixture to boiling (1-2 mins) (Cont. 4)
6. Mix ______________ for dispensing/clarifying.
7. Mix _______.
8. Filter the preparation while hot into the _________________________
9. Add _________ until it becomes 50mL
10. Stopper using __________. Let it cool in running water.
11. Add ___________
12. Stopper with ______ right away and trap the gas. Let bottle lie down to wet and expand the cork.
12 = ?
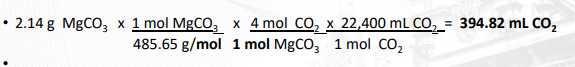
Calculation of Volume of CO2 produced from 2.14 g MgCO3
(MgCO3 )4 .Mg(OH)2 .5H2O + 5H3C6H5O7 → 5MgHC6H5O7 + 4CO2 + 11H2O
MW: (MgCO3 )4 .Mg(OH)2 .5H2O = 485.65 g/mol

Calculation of Volume of CO2 produced from 0.36 g KHCO3:
3KHCO3 + H3C6H5O7 → K3C6H5O7 + 3CO2 + 3H2O
MW KHCO3 = 100.115 g/mol
white
white or red label? magnesium citrate oral solution
120 mL wide mouth flint bottle
container for magnesium citrate oral solution
chemical reaction
method used in magnesium citrate oral solution
Sterilizing
____________ the bottle is done to prevent the growth of microorganism in the magnesium citrate oral solution.
cork or rubber liner of the cap is kept moist or swollen
Mg citrate solution is stored in a cold place, preferably in a refrigerator, keeping the bottle on its side so the ______________________________________, thereby maintaining the airtight seal between the cap and the bottle
deposit a crystalline solid upon standing
Magnesium Citrate Oral Solution is a troublesome solution as it has the tendency to _____________________________________________ due to the formation of some almost insoluble normal Mg citrate.