Chapter 18- Oxidative Phosphorylation
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Cellular respiration
Drives ATP formation by transferring electrons to molecular oxygen
Respiratory chain (electron transport chain)
Four large protein complexes that are embedded in the inner mitochondrial membrane
Oxidative phosphorylation
Set of electron-transfer reactions that captures the energy of high-energy electrons from NADH and FADH2
takes place in the electron transport chain
ultimately generates ATP and reduces oxygen to water
cellular respiration
cellular respiration
The generation of high-transfer potential electrons by the citric acid cycle, their flow through the respiratory chain, and the accompanying synthesis of ATP
Coupling of electron carrier oxidation and ADP phosphorylation
The flow of electrons from reduced carriers such as NADH is highly exergonic
NADH + 1/2O2 + H+ → H2O + NAD+
Favorable
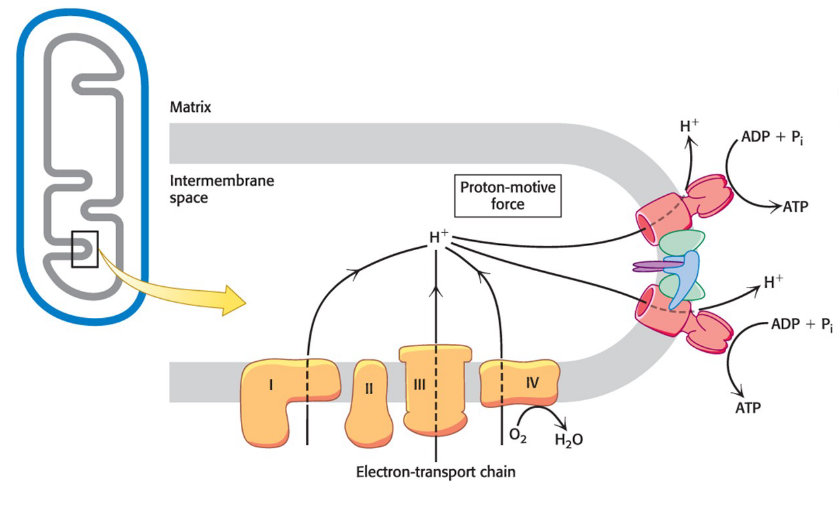
complexes of the electron-transport chain use released energy to pump protons out of the mitochondrial matrix
generates a pH gradient and a transmembrane electron potential that creates a proton-motive force that is used to power the synthesis of ATP
ADP + Pi + H+ → ATP + H2O
unfavorable
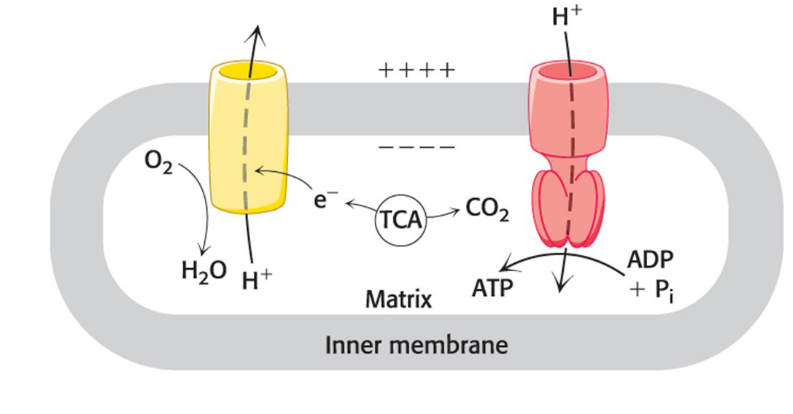
Mitochondria structure
The citric acid cycle, the electron-transport chain, and ATP synthesis occur in the mitochondria.
Mitochondria have two membranes (which creates two distinct internal compartments)
an outer membrane with porins
an extensive, highly folded inner membrane
intermembrane space (IM space) = compartment between the outer and inner membranes
matrix = compartment bounded by the inner membrane
Where do most citric acid and fatty acid oxidation reactions take place?
Mitochondrial matrix
Where does Oxidative phosphorylation take place?
Inner mitochondrial membrane
What does the respiratory chain consist of?
Four complexes: three proton pumps and a physical link to the citric acid cycle
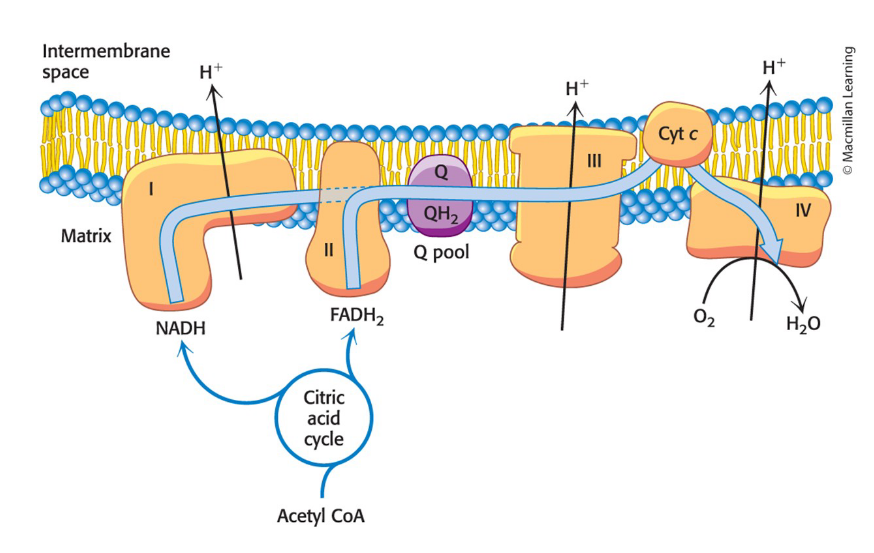
electrons flow from NADH to O2 through three protein complexes embedded in the inner mitochondria membrane
electron flow through complexes I, III, and IV is highly exergonic and power generation of a proton gradient
Complex I, III, and IV are proton pumps
Complex II
contains succinate dehydrogenase from the citric acid cycle
electrons from this FADH2 enter the electron-transport chain at Q-cytochrome c oxidoreductase
It does not pump protons
Pathway of electrons through the complexes
Complexes I, III, and IV appear to be associated in a supramolecular complex
facilitates the rapid transfer of substrate
Prevents the release of reaction intermediates
KNOW GRAPH
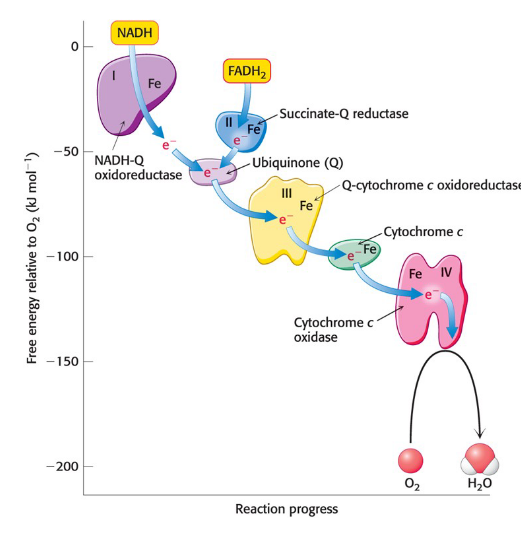
cytochromes
electron-transferring proteins that contain a heme prosthetic group
Electron transfer through NADH-Q Oxidoreductase is coupled to…
Proton transfer reactions
What is the entry point for electrons from FADH2 of flavoproteins
Ubiquinol
Complex II-
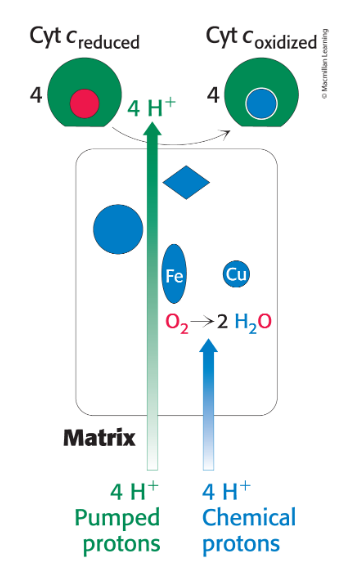
cytochrome c oxidase (Complex IV)
Catalyzes the transfer of four electrons from four reduced molecules of cytochrome c to O2
does cytochrome c oxidase catalyze the reduction of molecular oxygen to water?
Yes
Two components of proton transport by cytochrome c oxidase
Four chemical protons reduce O2 to two H2O.
Cytochrome c oxidase uses free energy from this reduction to pump 4 H+ from the matrix into the intermembrane space
Electrons flow via two pathways through the electron-transport chain
How are toxic reactive oxygen species limited in the mitochondria?
scavenged by protective enzymes
partial reduction of O2 generates highly reactive oxygen derivatives called reactive oxygen species (ROS)
ROS are implicated in aging and a growing list of diseases
ROS include superoxide ion, peroxide ion, and hydroxyl radical
cytochome c oxidase does not release ROS by holding O2 tightly between Fe and Cu ions
What powers the synthesis of ATP?
A proton gradient
flow of NADH to O2 is an exergonic process
Synthesis of ATP is an endergonic process
ATP synthase (Complex V)
A molecular assembly in the inner mitochondrial membrane that carries out the synthesis of ATP
Chemiosmotic hypothesis
Proposes that electron transport and ATP synthesis are coupled by a proton gradient across the inner mitochondrial membrane
suggested that ATP formation is powered by a proton gradient
Proton-Motive Force
The energy-rich unequal distribution of protons across a membrane
consists of a chemical gradient and a change gradient
powers the synthesis of ATP
proton-motive force (delta p) = chemical gradient (delta pH) + charge gradient
How does ATP Synthase assist in the formation of cristae?
Cristae formation allows proton pumps to localize the proton gradient in the vicinity of the synthases, which are located at the tips of the cristae
enhances efficiency of ATP synthesis
Oxidative phosphorylation overview
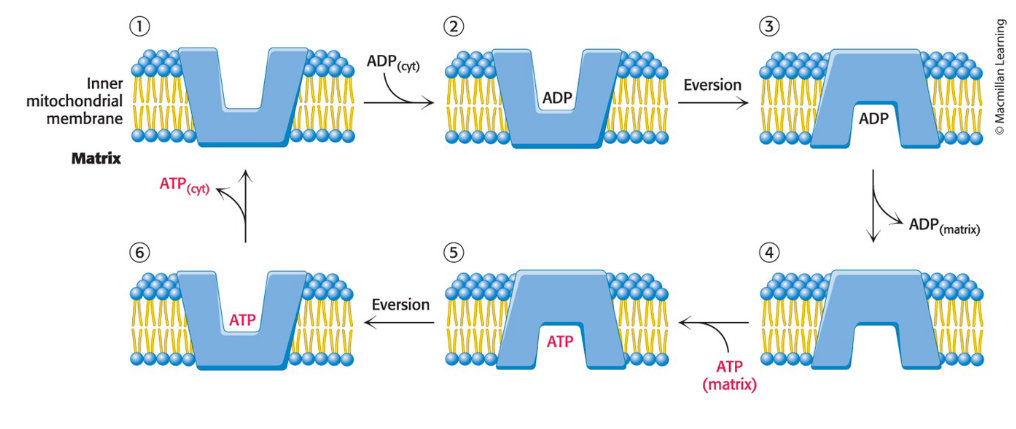
ATP-ADP translocase (adenine nucleotide translocase, ANT)
Specific transport protein that enables the exchange of cytoplasmic ADP for mitochondrial ATP
constitutes 15% of the protein of the inner mitochondrial membrane
ATP and ADP bind to ANT without Mg2+
Inhibition of ANT leads to the inhibition of cellular respiration
Entry of ADP into mitochondria is coupled to the exit of ATP by ATP-ADP translocase
ATP-ADP translocase catalyzes the exchange of entry of ADP and ATP
the translocase contains a single nucleotide-binding site that alternately faces the matrix and the cytoplasmic sides of the membrane
Regulation of cellular respiration
The ATP needs of the cell determine the rate of the respiratory pathways and their components
molecules of ATP formed when glucose is completely oxidized to CO2
Electrons do not flow through the electron-transport
chain unless ADP is available to be converted into ATPThe regulation of the rate of oxidative phosphorylation by ADP level is called respiratory (or acceptor) control.
At low ADP levels:
NADH and FADH2 are not consumed by the electron- transport chain.
the citric acid cycle slows because there is less NAD+ and FAD to feed the cycle.
Regulated uncoupling
Leads to the generation of heat
nonshivering thermogenesis = the ability to generate heat without using shivering by uncoupling oxidative phosphorylation from ATP synthesis
occurs in mitochondria-rich brown adipose tissue in animals
activated in response to a drop in the core body temperature
uncoupling protein 1 (UCP-1; also called thermogenin) = an inner mitochondria membrane protein that transports protons from the intermembrane space to the matrix with the assistance of fatty acids
generates heat by transporting protons without the synthesis of ATP
energy of the proton gradient, normally captured as ATP is released as heat as the protons flow through UCP-1 to the mitochondrial matrix